Intracranial Hypertension, Venous and Lymphatic Flow Dysfunction- the Core Hydraulic Dysfunctions
- Graham Exelby
- May 24, 2025
- 56 min read
Updated: Jun 13, 2025
Dr Graham Exelby May 2025
Abstract
Postural orthostatic tachycardia syndrome (POTS), particularly in the context of Long COVID, ME/CFS, and fibromyalgia, is increasingly understood as a manifestation of integrated autonomic, metabolic, and neurovascular dysfunction. One of the most debilitating but under-recognized components is dynamic intracranial hypertension (ICH), frequently missed by conventional supine-based diagnostics. This document delineates a comprehensive pathophysiological model of ICH grounded in impaired venous, cerebrospinal fluid (CSF), glymphatic, and lymphatic outflow—particularly in the upright posture. Evidence is drawn from neuroimaging, spectral CT, dynamic ultrasonography, and clinic-based anatomical analysis.
Key mechanisms include upright vertebral venous flow obstruction, cerebellar tonsillar descent (“plug-in-the-drain” phenomenon), fascial and lymphatic compression at the craniocervical junction, and disruption of astrocyte-mediated glymphatic clearance. These mechanisms converge to produce regionally elevated intracranial pressure and brainstem hypoperfusion, giving rise to hallmark symptoms such as orthostatic head pressure, pulsatile tinnitus, visual disturbances, “coat hanger” pain, and cognitive impairment. Special attention is given to the role of hypermobility syndromes (e.g., Ehlers-Danlos), IJVs at C1 and the base of the neck, and retrograde vertebral into emissary vein drainage.
This work redefines intracranial hypertension as a dynamic, posture-sensitive hydraulic disorder, with implications extending far beyond idiopathic variants. The evolving diagnostic and therapeutic landscape must pivot toward upright assessments and targeted interventions that restore integrated flow across venous, CSF, and lymphatic compartments. This refined framework offers a powerful lens through which to understand and address the complex symptomatology of POTS and allied syndromes at their mechanistic core.
Introduction
Postural orthostatic tachycardia syndrome (POTS) is a name for what is now a common condition in patients with Long COVID. The name was coined by Dr Phillip Low and Ron Schondorf at the Mayo Clinic in 1993 as a condition characterized by orthostatic intolerance (symptoms when standing) coupled with an increase in heart rate of at least 30 beats per minute for adults.(1)
POTS is a fusion of autonomic (dysautonomia), metabolic and inflammatory dysfunction driven by regional hypoxia. (Exelby 2025. (2)) The severity will vary dramatically- it is common in teens who often “grow out of it” after a couple of years. But this is not just a teenager’s intransient diagnosis, and patients have been seen in clinic from pre-teens into their 80’s.
POTS is not a psychiatric disorder, and 77% of POTS patients report being told they have a psychiatric condition, which increases the severity of symptoms. Patients with POTS can be misdiagnosed as having severe anxiety, panic disorder, or chronic fatigue syndrome, because of their similar clinical features.
Brainstem hypoxia represents a common pathway in a wide spectrum of neurocardiovascular and neuroimmune syndromes including POTS, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), Long COVID, fibromyalgia, and Gulf War Syndrome. The brainstem—particularly the medulla and rostral ventrolateral medulla (RVLM)—is critical for autonomic, cardiovascular, and respiratory homeostasis. (2)
Hypoperfusion in these regions triggers a cascade of maladaptive responses: excessive sympathetic activation, impaired baroreflex sensitivity, parasympathetic withdrawal, and altered respiratory rhythmogenesis. These features form the clinical core of many overlapping syndromes within the dysautonomia spectrum.(2)
Neuroimaging studies, including brain SPECT as used in clinic investigations, MRI, and PET imaging, have consistently demonstrated hypoperfusion in the brainstem, cerebellum, and frontal subcortical circuits in affected individuals. The molecular footprint of this hypoperfusion includes stabilization of hypoxia-inducible factor 1-alpha (HIF-1α), activation of pyruvate dehydrogenase kinase (PDK), mitochondrial dysfunction, and neuroinflammation driven by RAGE/NF-κB/CCL2 signalling.(2)
These processes contribute to the characteristic metabolic exhaustion, post-exertional malaise (PEM), and central sensitization seen in POTS, ME/CFS, and Long COVID. The varying patterns of hypoperfusion and hyperperfusion are demonstrated in brain SPECT scan changes described in Brain SPECT changes in POTS, CFS, FMS and Long COVID.(Exelby 2025 (3))
Emerging clinic observations suggest that PEM may be at least partially mediated by retention of hypoxic byproducts and neuroinflammatory metabolites within the extracellular matrix. Manual lymphatic drainage (MLD), particularly using protocols targeting cervical, axillary, and thoracic ducts, has been observed to rapidly relieve PEM, suggesting that impaired clearance of these metabolites is central to its persistence. This reinforces the role of lymphatic and glymphatic stasis in post-exertional symptomatology.
There is sound evidence suggesting a strong link between intracranial hypertension (ICH) and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), fibromyalgia (FM), Long COVID, Gulf War Syndrome and POTS. A study by Hulens et al 2023 (4) explored the shared pathophysiology between idiopathic intracranial hypertension, fibromyalgia, and chronic fatigue syndrome, suggesting a potential link among these conditions.
The distinctive symptoms of “brain fog with pressure” typifies one of the major symptoms experienced by people with POTS, Long Covid, fibromyalgia and Chronic Fatigue Syndrome. There are no accurate epidemiological studies to assess the correlation, migraine being reported by Ray et al 2022 (5) at 36.8% and general headache prevalence rates ranging up to 94% by Shaw et al 2019 (6))
Orthostatic headache data (Ray et al 2022 (5)) is more important as we believe this represents Intracranial Pressure Dysfunction with the predominant cause from a combination of venous and lymphatic outflow obstruction. Accompanying pulse-synchronous tinnitus and visual changes should point the clinician to consider Intracranial Hypertension (ICH), other potential causes such as CSF Leaks should be considered.
In the current modelling presented in this paper, head “pressure” can be divided into supine types where IJV obstruction dominates, standing when vertebral venous obstruction dominates, or both. Lymphatic / CSF flow obstruction is a major factor in both types. As pressure builds in the CSF, CSF leaks may occur, relieving the pressure for a short period. This can be quite apparent in Ehlers Danlos Syndrome patients with POTS. Ganesh & Munipelli 2024 (7) describe the close association with hypermobility spectral disorders in 30 to 57% of patients with ME/CFS, fibromyalgia, POTS, and Long COVID. This hypermobility may contribute to structural instabilities that could predispose individuals to ICH.
There are remarkable similarities in potential symptoms in ICH and brainstem hypoperfusion, and while clinic investigations reveal often totally different apparent causes, they are still intricately linked. Increasingly we are drawn to source underlying causes, and their complex interaction. In the symptomatic patients there are a number of “moving parts” affecting the mechanics of the neck, thoracic outlet and the hydraulics involving arteries, veins and lymphatics, particularly, but not exclusively of the head and neck that need to be examined individually and together.
A Starting Point in POTS
When working through the complex pathogenesis and how these create the symptoms that reflect POTS, there are some that provide clues to underlying causes, and symptoms persist for at least 3 months:
Fatigue- where in the formalised version from NICE guidelines, incorporates:
Debilitating fatigue worsened by activity, is not caused by excessive cognitive, physical, emotional or social exertion, and is not significantly relieved by rest.
Post-exertional malaise -after activity where the worsening of symptoms: (described like the battery-operated bunny when it runs out of battery power”)
Is often delayed in onset by hours or days
Is disproportionate to the activity
Has a prolonged recovery time that may last hours, days, weeks or longer
Unrefreshing sleep or sleep disturbance (or both), which may include:
feeling exhausted, feeling “flu-like” and stiff on waking.
broken or shallow, altered sleep pattern or hypersomnia
Cognitive difficulties (sometimes described as 'brain fog'), which may include problems finding words or numbers, difficulty in speaking, slowed responsiveness, short-term memory, and difficulty concentrating or multitasking.
Other symptoms characteristic of POTS:
Sensitisation
Post-exertional malaise
Autonomic instability (dysautonomia)
Coat hanger pain
Headaches- which may be described in various ways, and may include migraine +/- aura, but for many patients, reflects intracranial pressure dysfunction in the venous and CSF/lymphatic systems.
Intracranial Hypertension and Venous Obstruction: Background
It is when looking closely at the headache types and patterns that Intracranial pressure dysfunction that can result in Intracranial Hypertension (ICH) can be seen. At present ICH investigation and treatment has focussed on supine pressure, and the key diagnostic tool has been CSF pressure from lumbar puncture. But this system is not static, and is a dynamic system that may be normal in the supine position where CSF pressures are taken, and increased CSF opening pressure is likely to be normal or in an indeterminate range.
Intracranial hypertension (ICH), driven by hydraulic dysfunction, forms a central mechanism linking HPA axis dysfunction, glycaemic imbalances, PDH dysfunction, and glymphatic impairments. These interconnected systems underlie many of the symptoms seen in POTS, Long COVID, and fibromyalgia.
Intracranial pressure symptoms reflecting Intracranial Hypertension is increasingly seen to be driven by venous obstruction, and the sites where this obstruction occurs also provides clues to accompanying lymphatic obstruction. The primary venous outflow when supine is via the Internal Jugular Veins,(IJV) and when standing, the vertebral veins. In both, lymphatic obstruction can be seen, although at this point usually only with clinical observation. But together they form a complex hydraulic dysfunction that reflects the typical head pressure symptoms.
The vertebral venous flow dysfunction may also link the intracranial hypertension both supine and erect, and may explain the progressive cerebral hypoperfusion seen in these patients, adding to the proposed hypotheses of sympathetic vasoconstriction and reduced arterial flow.
There are difficulties in confirming the increased CSF pressure in the standing subgroup, as the MRI findings may not confirm this dynamic situation. Current standing MRIs lack the definition to see this. CSF leaks can also be difficult to confirm on MRI, as for many patients with the small leaks, they act as “relief valves,” and only when standing.
The biggest clinical difficulty is separating the CSF/lymphatic/glymphatic obstruction from venous obstruction and its secondary lymphatic obstruction. Pulsatile tinnitus provides a major clue. The lymphatic therapy researcher Michelle Hill feels venous backflow is the more likely primary problem, but as yet we cannot image this.
When CSF leaks are found, clinic studies of history often suggests increasing head pressure followed by the CSF leak which relieves it. Symptoms may vary and while frequently be more suggestive of Intracranial Hypotension, this is not supported by patient data, as these appear to be secondary to craniovascular pressure changes and an inestimable number of these have CSF leaks. Yousry et al (8)) found focal fluid collections in the retro-spinal region at C1-2 in their investigations of postural headache.
High quality neuroimaging is critical in sorting these patients out, and read by neuroradiologists aware of the nuances of the findings they might see.
Retinal photography provides an excellent tool, not only showing the increased pressure, but also changes in retinal vasculature. Changes are very common, but are usually subtle with increased AV tortuosity.
Figure 1. Retinal Photography
High level retinal photography + OCTs can provide valuable information on both vascular and CSF flow. This image shows AV tortuosity with a microhaemorrhage

Source: Alan Ming Optometrist
Figure 2. Empty Sella

Source: Jones, J (revised) Empty Sella. Radiopedia 2025. https://radiopaedia.org/articles/empty-sella
Figure 3. Optic Nerve Tortuosity

Courtesy of Bouhouche Abdeldjalil, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>.
For many patients, a few simple questions- whether there is head pressure, where it is, and if standing or lying, whether there is pulsatile tinnitus, and what visual changes occur can provide most of the answers. This is a dynamic hydraulic system. Timing and position of the head pressure -notably when supine or standing, occipital or frontal or combinations, especially when accompanied by visual changes and pulsatile tinnitus are critical clues. It is very important the time taken for symptoms to present.
Understanding the dynamic nature of CSF, lymphatic and venous outflow from the brain provides an insight into the ICH symptoms seen in POTS, Long COVID and Fibromyalgia. Migraine may co-exist, and indeed dual pathology is very common clinically, and consideration for CGRP inhibition may need to be considered. Once this is established, then the clinician should move to what is causing the symptoms.
Lymphatic Clearance and the Resolution of Post Exertional Malaise (PEM)
In select clinical cases, targeted manual lymphatic therapy protocols focused on opening cervical, axillary, parasternal, and thoracic lymphatic territories have led to rapid resolution of interscapular oedema and associated PEM. These findings suggest that PEM reflects a state of lymphatic-ECM congestion, likely enriched in hypoxic intermediates, inflammatory cytokines, and lactic acid metabolites.
Restoration of lymphatic flow facilitates metabolic washout, reduces cerebral and paraspinal oedema, and correlates with normalization of GABA and aspartate profiles in urine. This supports a paradigm wherein PEM may not solely reflect mitochondrial failure, but rather ECM-bound toxin retention due to lymphatic flow obstruction.
The current known pathogenesis of intracranial hypertension (ICH) encompasses various mechanisms, broadly categorized into primary ICH (idiopathic intracranial hypertension, IIH) and secondary ICH, which may result from identifiable causes, including venous, glymphatic, CSF and lymphatic obstruction. I believe there is an underlying cause in all, making “idiopathic” a term that says you haven’t looked hard enough.
The cerebrospinal fluid (CSF) circulation and its dynamics within the recently identified CSF canalicular system (ventricular and subarachnoid compartments) and the meningeal lymphatic system play critical roles in maintaining brain homeostasis. Postural changes have a significant impact on these systems due to gravitational influences on fluid dynamics. Various mechanical factors come into play affecting both cerebral venous outflow and CSF flow dynamics.
In the symptomatic patients there are a number of “moving parts” that need to be examined individually and collectively. By looking at potential causes of symptoms, rather than be overwhelmed by their complexity, or simply look at medication to control the tachycardia and other symptoms, the underlying “drivers” can usually be worked out and may be successfully managed. Lumbar puncture and “blind” CSF blood patches are not without risks so should be used when clinicians are confident of exactly what they are treating.
Breakthrough vascular discoveries- Retrograde cervical vertebral venous retrograde flow-“Plug in a Drain.”
Spectral CT angiography/venography developed at Mermaid Beach Radiology by Dr Zane Sherif has been able to demonstrate low lying cerebellar tonsils with aqueduct obstruction that are not seen on MRI. The diagnosis of “low lying cerebellar tonsils” is seems to be clouded with a degree of uncertainty, and inadequate care on the radiologist reporting. In a “marginal” call the Aqueduct of Sylvius, a narrow channel connecting the third and fourth ventricles facilitating the flow of CSF may provide answers, but these too are dependent on the clinician referring the patient to alert the radiologist to this suspicion of dysfunctional CSF flow.
Figure 4. The Cerebral Aqueduct (Aqueduct of Sylvius)

Source: By Henry Vandyke Carter - Henry Gray (1918) Anatomy of the Human Body -Gray’s Anatomy, Public Domain, https://commons.wikimedia.org/w/index.php?curid=541556
Initially this seems a paradox until realisation shows that the Spectral CT is so fast it may capture this before it has had time to “slip back into position” in the MRI, a much longer procedure. This is particularly apparent in the subgroup with low lying cerebellar tonsils, for in this group, the erect posture allows the cerebellum to settle into the foramen magnum, obstructing both venous and lymphatic flow, to be relieved when lying. (Heiss 2012 (9)) I describe this phenomenon as a “plug in a drain,” and a critical component of the standing head pressure in POTS and Long COVID.
Dynamic ultrasounds developed by vascular sonographers Mss Rylee White and Danielle Adey from Australian Ultrasound Specialists at the Gold Coast, of cervical vertebral venous flow in patients where there is increased pressure when standing (seated) has found a very high incidence of impaired venous flow, especially with postural changes, such as head flexion with rotation. There may also be increased obstruction of the Internal Jugular Veins compounding the venous and lymphatic obstruction. (Muccio et al 2024. (10))(Williams 2008 (11))
A significant number have retrograde venous flow which could not explained until the “plug in a drain” phenomenon has been radiologically confirmed, causing progressive obstruction to venous and lymphatic flow, behaving like a Chiari malformation, and exaggerated in patients with Ehlers Danlos Syndrome.
It is most likely the retrograde flow is venous flow into the emissary veins, which while standing, continue to drain. Williams 2008 (11)) described impaired IJV drainage can force blood into emissary veins, showing retrograde flow. It appears likely that both IJV and cervical vertebral vein obstruction are required for this phenomenon. Studies to confirm this phenomenon are continuing.
Upright postures reduce cervical CSF flow by over 50% compared to supine positions, limiting fluid exchange between the cranium and spinal canal.(Muccio et al 2024 (10)) This impairs waste clearance via glymphatic pathways, which rely on CSF-ISF (interstitial fluid) exchange. (10)(11) In Chiari patients, obstructed CSF flow at the foramen magnum disrupts pressure gradients necessary for glymphatic transport, potentially accumulating neurotoxic waste. (9) Moving from supine to upright positions causes a rapid ICP drop in healthy individuals, but Chiari patients exhibit a blunted response due to compromised CSF outflow. Sustained ICP elevation in upright postures may compress lymphatic vessels, further hindering drainage. (11)
Figure 5. Low Lying Cerebellar Tonsils

Scan provided with patient permission
Figure 6. Cerebellar Tonsillar Position.
The cerebellar tonsillar position is the distance (yellow line) measured from the tip of the cerebellar tonsils to a line between the anterior and posterior rims of the foramen magnum (McRae Line- blue line) at right angles. Normal tonsils are above the foramen magnum but it may descend a few millimetres without constituting a Chiari 1 malformation.

Source: Gaillard, F. Cerebellar Tonsillar Position. Radiopedia. https://radiopaedia.org/cases/cerebellar-tonsillar-position-illustration
It is to be expected this would be particularly apparent in the subgroup with low lying cerebellar tonsils, for in this group, the erect posture allows the cerebellum to settle into the foramen magnum, obstructing both venous and lymphatic flow, to be relieved when lying, so may not been seen on MRI.
The speed of onset of head pressure standing can be quite variable, reflecting the anatomy and hydraulic obstruction involved in each patient.
The cerebellar tonsils exhibit dynamic positional variability influenced by gravitational forces, intracranial pressure (ICP) gradients, connective tissue integrity, and CSF compliance. In the upright posture, cerebrospinal fluid redistributes inferiorly, and venous outflow from the cranial vault shifts toward the vertebral and paraspinal systems.
In individuals with impaired craniospinal compliance or connective tissue laxity—as seen in Ehlers-Danlos Syndrome—this redistribution can precipitate inferior displacement of the cerebellar tonsils into the foramen magnum. This descent is mechanically exacerbated by loss of cerebellar support, tethering of the spinal cord or brainstem, and transient reductions in subarachnoid CSF volume during orthostasis.
The result is a functional obstruction at the craniocervical junction, compromising CSF and venous outflow while increasing local tissue pressure. This dynamic process, often missed on supine MRI, can induce symptoms mimicking Chiari I malformation despite absence of fixed tonsillar herniation.
The transient “plug-in-the-drain” effect, reveals a biomechanical failure of vertical suspension and drainage that contributes to upright intracranial hypertension and brainstem compression syndromes.
Fourth Ventricle, Hypoxia, and Autonomic Disassociation
Emerging neurophysiological studies, including those described by Peter Juli (unpublished, 2025, personal communication), suggest that disassociation between central autonomic nuclei and respiratory control centers in the vicinity of the fourth ventricle may play a pivotal role in hypoxic sensitivity and sympathetic dysregulation. This region, anatomically surrounded by the dorsal vagal complex, nucleus tractus solitarius (NTS), and area postrema, acts as an integrative hub for chemosensory input and cardiorespiratory feedback.
Experimental data from rodent models and in vitro slice physiology (e.g., Lamy & Laguzzi, Brain Res Bull, 2020) have shown that modest hypoxia within the floor of the fourth ventricle can decouple vagal tone and sympathetic restraint, promoting a hyperadrenergic state. Importantly, CSF dynamics in this compartment are gravity-sensitive and susceptible to mechanical distortion from cerebellar tonsillar descent and craniocervical compliance abnormalities, consistent with the "plug-in-the-drain" model described earlier in this manuscript.
Juli's proposed framework suggests that upright posture induces transient perfusion mismatch and CSF stagnation within the fourth ventricle, compromising its role in synchronizing afferent cardiovascular and respiratory signals. This is supported by studies showing ultra-slow BOLD fluctuations in the fourth ventricle correlating with vigilance states and hypoxic responsiveness (Yuan et al., Neuroimage, 2022).
This disassociation may be particularly prominent in patients with orthostatic intolerance and those exhibiting excessive cerebellar descent, contributing to sympathetic surges, disordered breathing, and paradoxical hyperventilation commonly seen in POTS and Long COVID. From a mechanistic perspective, impaired clearance of CO2 and local pH fluctuations in the ventral medullary surface could exacerbate baro- and chemoreflex failure, further destabilizing autonomic tone.
Clinically, this adds another layer to the understanding of upright ICH, whereby the fourth ventricle is not only a CSF conduit but a vulnerable zone for dynamic hypoxia-induced autonomic disintegration
Biomechanics of Cerebellar Tonsillar Descent in Upright Posture:
MRI studies demonstrate cerebellar tonsils descend further in upright vs. supine positions, particularly in conditions like adolescent idiopathic scoliosis. Mean tonsillar excursion in AIS patients was -1.9 mm (±2.3 mm) compared to -0.1 mm (±0.2 mm) in controls, highlighting gravitational influence.(Lee et al 2015 (12)) The implications are that upright imaging captures positional variability missed in supine scans, critical for diagnosing functional obstruction, (12) but I believe this is severely limited by inherent MRI quality.
ICP increases by 3–9 mmHg when the head is turned or tilted in upright postures due to jugular venous compression and altered CSF dynamics. This elevates craniospinal pressure gradients, exacerbating tonsillar descent.(Bancroft et al 2025 (13)) Upright posture reduces cervical CSF flow by >50%, impairing glymphatic clearance and increasing subarachnoid space pressure. (13)
Craniocervical instability in EDS reduces dural compliance via mechanical overload of the myodural bridge complex. Stiffened dura amplifies CSF pulse pressure during cardiac cycles, worsening tonsillar herniation. Trauma or hypermobility in EDS disrupts vertical suspension of the cerebellum, allowing gravity-dependent tonsillar descent.(Spiessberger et al 2020 (14)) The connective tissue laxity in EDS patients worsens tonsillar obstruction and venous compliance, amplifying retrograde flow risks.
Internal jugular veins collapse in upright positions, shifting drainage to vertebral/paraspinal veins. Retrograde flow into emissary veins compensates but increases intracranial venous pressure.(13)
The core pathophysiological elements are:
1. Mechanical Obstruction at the Cranio-cervical Junction:
With upright posture, cerebellar tonsils herniate slightly into the foramen magnum, causing a "plug-in-a-drain" effect.
This transient obstruction increases resistance to cerebral venous and lymphatic outflow, mimicking a functional Chiari malformation.
EDS-associated ligamentous laxity may exaggerate brainstem and cerebellar "settling" in upright posture.
2. Retrograde Flow and Emissary Vein Reversal:
Obstruction leads to retrograde venous flow through the cervical vertebral venous plexus and emissary veins, which under normal physiology act as outflow safety valves.
The reversal is pathological and reflects increased intracranial venous pressure, particularly during head rotation/flexion, worsening drainage.
3. Lymphatic Flow Impairment:
The glymphatic and extracranial lymphatic drainage systems, particularly reliant on pulsatility and posture, may be compromised.
Stagnant interstitial fluid and impaired toxin clearance, including lactate and neuroinflammatory mediators, may contribute to brain fog, pressure, and neuroinflammatory states.
4. Intermittent Intracranial Hypertension & Brainstem Compression:
Dynamic compression may induce transient intracranial hypertension, and possibly chronic ischaemia or hypoperfusion in the brainstem, cerebellum, and cortex.
5. Autonomic and Neurovascular Dysregulation:
With venous outflow resistance, venous congestion may impair the baroreceptor and chemoreceptor feedback loops (notably via the nucleus tractus solitarius, locus coeruleus, and vestibular nuclei), contributing to orthostatic intolerance.
Anticipated Raised Intracranial Pressure-like Symptoms from “Plug in a Drain” -(Especially when standing or with neck flexion/rotation):
Head pressure (worse upright, relieved when lying down)
Occipital pain or fullness
"Leaning forward" or "tight helmet" sensation
Visual changes: transient blurred vision, photophobia
Pulsatile tinnitus
Brainstem & Cerebellar Involvement (Chiari-like):
Vertigo or ataxia
Dysautonomia/POTS-like symptoms
Swallowing difficulty or throat tightness
Vocal changes
Palatal myoclonus or facial twitching
Occipital neuralgia
Cognitive & Neuroinflammatory Symptoms:
Brain fog
Memory and concentration difficulties
Fatigue
Insomnia
Autonomic Dysfunction
POTS or orthostatic hypotension
Syncope or near-syncope
Palpitations
Hyperadrenergic states (due to impaired baroreflex)
Respiratory Dyscoordination (from brainstem/cervical effects):
Air hunger
Paradoxical breathing
Sleep apnoea-like symptoms
Exacerbated by:
Standing upright
Head flexion, rotation (esp. if cervical venous reflux is provoked)
Valsalva or exertion
Dehydration or low preload states (e.g., hypovolemia)
These findings provide evidence of intracranial venous and CSF obstruction, allowing an understanding of Intracranial Hypertension in an erect position, extending from the traditional supine-only diagnosis, and an why CSF opening pressures may be normal in these patients.
The retrograde vertebral venous flow implicates dysfunction in the vertebral and IJV venous systems, leaving the emissary veins in the neck to deal with the venous outflow, which it simply cannot adequately control.
Figure 7. Posterior view of venous structures in suboccipital and upper cervical region
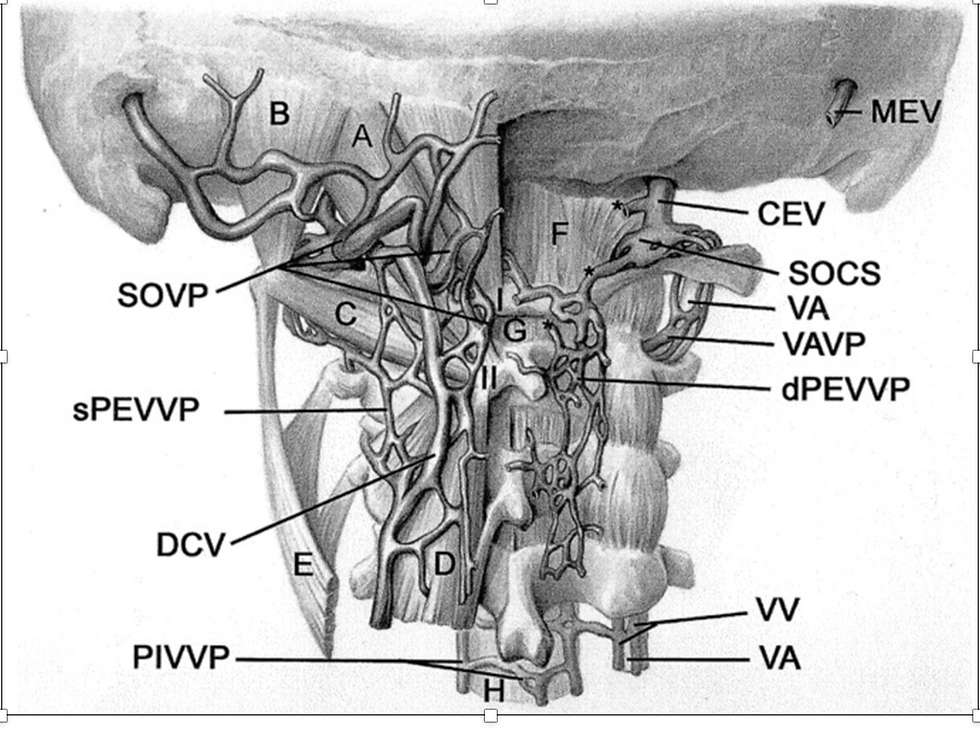
Source: Yousry et al 2001. Cervical MR imaging in postural headache: MR signs and pathophysiological implications.(8)
Figure 8. Coronal Cross Section of Skull showing Emissary Vein Uppermost and Arachnoid Granulation

Source: Wikipedia. Emissary Vein. https://upload.wikimedia.org/wikipedia/commons/thumb/5/5f/Gray769-en.svg/704px-Gray769-en.svg.png?20220218113714
The emissary veins connect the extracranial venous system with the intracranial venous sinuses. There are several types of emissary veins including the posterior condyloid, mastoid, occipital and parietal emissary veins.
Clinic therapists report a ”bogginess” in the occipital region, and related to functional neck positioning which we believe this is responsible for the majority of these findings.
There is a wide variability in speed on onset of this ICH, at time immediate on standing, in others a slow development that can be traced to posture and things like sustained computer use. These slow onset types may be corrected by a “rest” before the anticipated onset of symptoms. Others are more complex and require a close examination of the individual hydraulics and neck, TOS, IJV and lymphatic architecture.
Figure 9: CT Reconstruction of Venous Collaterals in a Vertebral Venous Obstruction

Source: Reconstruction by Phillip Russell from CT by Dr Zane Sherif with patient permission
Symptoms of Intracranial Hypertension (ICH)
The distinctive symptoms of “brain fog with pressure” accompanied by “brain fog” typifies one of the major symptoms experienced by people with POTS and similar problems, especially Long Covid and Chronic Fatigue Syndrome. Accompanying pulse-synchronous tinnitus and visual changes should point the clinician to consider Intracranial Hypertension (ICH). Not all POTS fit the classical symptoms of ICH, or even CSF leaks. Increasingly we are drawn to other potential causes, and the complexity of the neuroinflammatory dysfunction in Long COVID requires a detailed assessment of exactly what is happening in each patient.
Symptoms will vary, and not every POTS has the pressure headaches, or visual changes, but so many are simply missed, or labelled as tension or neck-related headaches or migraine. The partial success of botulinum toxin into the sub-occipital muscles provides an example of symptom treatment rather than dealing with underlying causes.
Main symptoms include:
Headache, head pressure, or head pain, as with low pressure, is a key symptom of ICH. Intracranial pressure headaches may not be relieved by pain medication. The pain may be located behind the eyes, forehead, one side of the head, back of the head, base of the skull, top of the head, and maybe burning or pressure-type pain, and may be made worse by eye movement. Any head pain related to pressure or positional change is important.
Pulse-synchronous tinnitus (whooshing, whistling, humming, or marching noises in one or both ears that correlates with your heartbeat). This is strongly linked to flow changes in the brain’s venous system creating turbulence transmitted to the cochlea.(see below)
Light and scent sensitivity worsening with exertion, cough, or straining (as central sensitisation increases)
Vision changes—ICH can cause rapid or progressive vision changes which may include grey spots, dots, floaters, or dim-outs in one or both eyes, blurred vision, or double vision.
Tables 1 to 4 describe symptoms attributed to the various causes of pressure change -some separated only by the symptoms of postural change.
Table 1. Symptoms of Intracranial Hypertension, Intracranial Hypotension and CSF Leaks
Intracranial Hypertension
(7)(15)(16 | Spontaneous Intracranial Hypotension (7)(8)(17) | CSF Leak
(18)(19) | Intracranial Venous Hypertension
(7)(20)(21(22)(23) |
-Headaches, usually worse in mornings and lying (may not respond to analgesics, and may be worse with eye movement) -Visual disturbances, eg blurred vision, double vision, loss of peripheral vision, “dim outs”, grey spots, flashes in visual field -Pulsatile tinnitus -Whooshing / whistling in time with pulse -Nausea and vomiting -Dizziness -Neck pain and stiffness Other symptoms (6)
-Fatigue -Excessive sleepiness -Brain fog, cognitive impairment -Mood or behavioural changes -Weakness, speech difficulty -Numbness, paraesthesiae hands, feet or face -Malaise -Exercise intolerance |
-Positional headache, which worsens when you sit up and improves when you lie down. -Neck pain/stiffness -Back/ interscapular pain -Muffled hearing -High-pitched tinnitus -Vertigo -Ataxia -Blurred vision -Diplopia -Photophobia -Nystagmus -Cognitive slowing -Impaired memory, attention span -“Brain fog” -Fatigue -Lightheadedness -Facial sensory disturbance
|
-Positional headache, worse upright -nausea and vomiting --Sensitivity to light or sound. -Nausea, with or without vomiting. -Neck pain or stiffness. -Hearing changes, such as muffled hearing or ringing in the ears. -CSF drainage from nose, back of throat, ear, sinus tracts -loss of smell -Salty taste mouth
Other presentations (24) -Dementia -Parkinsons / movement disorders -Stroke -Cerebral vasoconstriction -Spinal symptoms
|
-Headache -Papilloedema/ retinal vascular changes -Visual symptoms (diplopia, blurred vision,grey spots, “dim-outs”) -Tinnitus -Pulse-synchronous tinnitus -Elevated CSF pressure -Dizzyness -Hearing impairment -Sleep disorder -Anxiety / depression -Cognitive change |
Table 2. Symptoms of Craniovascular Hypertension, Brainstem hypoperfusion and Vertebral Artery Hypoplasia
Craniovascular Hypertension (25) | Brainstem Hypoperfusion (26) | Vertebral Artery Hypoplasia (27) |
-Headache (pulsating) -Eye pain -Seizures -Tinnitus -Blurred vision, visual aura -Dizzyness -Nausea and vomiting -Confusion |
-Fatigue -Cognitive dysfunction (brain fog) -Sleep disorder -Increased pain sensitization -Autonomic dysregulation -Orthostatic intolerance, POTS -Dizzyness -Vertigo -Limb weakness (paralysis) -Difficulty swallowing -Difficulty speaking -Syncope -Sleep Apnoea -Mood disorder |
-Vertigo/dizziness/ ataxia, loss of coordination -Difficulty speaking / swallowing -Visual changes- partial or complete loss one or both eyes -Difficulty swallowing, or speaking as brainstem affected -Occipital headaches-often throbbing or pulsating, accompanied by neck pain -Cranial nerve dysfunction such as eye movement, facial sensation, and hearing. -Cognitive and memory problems: Severe cases of vertebral artery obstruction can lead to cognitive impairment, including memory problems, difficulty concentrating, and confusion. |
Table 3. Symptoms of Transverse, Sigmoid, Sagittal Sinus Obstruction and Aberrant Arachnoid Granulations
Transverse Sinus Obstruction and Sigmoid Sinus Obstruction (continuation of Transverse Sinus) (28) | Sagittal Sinus Obstruction
(29) | Aberrant Arachnoid Granulations
(15)(30)(31)(32)(33)(34) |
- Headache- throbbing and back of head - Pulsatile tinnitus -Seizures -Focal neurological deficits -Altered mental state -Visual change, eg blurred -Syncope -Nausea and vomiting -Increased Intracranial Pressure
|
-Headaches -Altered mental state -Seizures -Visual disturbances-blurred -Nausea and vomiting -Weakness -Increased Intracranial Pressure |
-Headaches (pressure/throbbing) worse straining and postural change CSF leakage -Nausea and vomiting -Visual disturbances- blurred, optic disc oedema -Neck pain, stiffness -Ataxia -Positional dizziness -Auditory changes -Pulsatile Tinnitus -Cognitive impairment -Intracranial Hypertension |
Table 4. Symptoms of Lymphatic and Glymphatic Obstruction, and “Plug in the Drain”
Lymphatic obstruction at Foramen Magnum
(9)(35)(36)(37)(38)(39)(40) | Lymphatic Obstruction at angle of jaw (Eagle/Stylohyoid Syndrome) (41)(42)(43) | Glymphatic Obstruction
(44)(45) | “Plug in the Drain” -venous and lymphatic obstruction at Foramen Magnum |
-Chronic headaches, worse straining esp pulsatile -Upper neck pain from increased intracranial pressure or CSF flow -Ataxia Cognitive impairment -Involuntary tremors -Loss of muscle tone in extremities (compression in cerebellum or brainstem) -Cranial nerve dysfunction- including difficulty swallowing, sensory changes, visual disturbances -Swelling /pain around face, neck and throat -Weakness limbs loss fine motor control, paraesthesia from spinal cord compression or syringomyelia -Fatigue -Neuroinflammation -Increase autoimmune activation
Associated Conditions -Chiari malformation -Foramen Magnum tumours -Lymphatic dysfunction |
-Throat pain, especially swallowing -Ear pain -Foreign body in throat -Difficulty moving tongue -Difficulty opening mouth fully -Neck pain radiating to jaw or ear Other Eagle Syndrome symptoms -Headache, behind eyes, worse coughing, straining, in mornings -Visual changes- blurred, visual changes, diplopia Loss peripheral vision Tinnitus- pulse synchronous -Nausea and vomiting -Eye pain |
-Neuro-ocular symptoms Cognitive impairment -Increased risk neuro-degenerative disorders, -increased motor symptoms in Parkinsons disease -Increased disease progression in ALS -sleep disorder -Increased intracranial head pressure Activation of microglia and astrocytes increasing inflammation |
-Head pressure, sores upright, relief lying -Occipital pain or fullness -“Tight helmet” leaning forward -Visual changes- blurred vision, photophobia - Ataxia / vertigo -Brain fog/ Cognitive impairment -Dysautonomia Swallowing difficulty/throat tightness -Vocal changes -Facial twitching -Occipital neuralgia Fatigue -Insomnia -Syncope/pre-syncope -Palpitations -Hyperadrenergic state (baroreflex impairment) -Air hunger -Paradoxical breathing -Sleep apnoea
|
Table 5. MRI findings of Intracranial Hypertension, Intracranial Hypotension and CSF Leaks
Intracranial Hypertension (7)(9)(10) | Intracranial Hypotension (7)(15)(16) | CSF Leak
(46)(47)(48) | Intracranial Venous Hypertension (7)(20)(21)(22) |
-Increased paravascular space -Empty sella turcica -Flattening of posterior globes of eyes -Optic Nerve sheath distension -Aberrant arachnoid granulations -Venous sinus thrombosis -Venous sinus focal stenosis
|
-pachymeningeal enhancement -distension of venous dural sinuses -intracranial venous thrombosis -enlarged pituitary gland -subdural effusions/haematomas -downward displacement of cerebral structures
Spinal MRI Findings -dilatation of venous plexus -spinal hygromas -retrospinal fluid collections (transudate from venous plexus) -dilated perineural root sleeves |
In EDS, none of these classical signs may be apparent
-Aberrant arachnoid granulations -subdural fluid collections -Cerebral venous thrombosis |
-Increased paravascular space -Empty sella turcica -Flattening of posterior globes of eyes -Optic Nerve sheath distension -Aberrant arachnoid granulations -Venous sinus thrombosis -Venous sinus focal stenosis
|
Pulsatile Tinnitus
Pulse-synchronous tinnitus (PT) (whooshing, whistling, humming, or marching noises in one or both ears that correlates with your heartbeat). This is strongly linked to flow changes in the brain’s venous system creating turbulence transmitted to the cochlea.
Research describes the following potential causes of PT, commonly seen in POTS on MRI venography , Spectral CT angiography/venography and brain SPECT or the newly developed MRI NeuroQuant Studies:
Vascular anomalies and turbulent flow, often with multiple lesions - like transverse sinus stenosis (TSS), dehiscent sigmoid plate, and jugular bulb anomalies (e.g., diverticulum, dehiscence) are prevalent in venous PT patients. These anomalies disrupt laminar blood flow, creating turbulence that is transmitted to the cochlea. Multiple abnormalities may coexist often coexist: 70% of patients have ≥2 vascular lesions (e.g., TSS + sigmoid sinus diverticulum), amplifying turbulence and PT severity. (Dong et al 2015 (49)) ( Essibayi et al 2021.(50)) (Abdalkader et al 2021 (51))
Reduced cerebral blood flow especially in cerebellum and temporal lobe, restored by transverse sinus stenting. (Li et al 2021 (52))
IJV obstruction, where stenting relieved PT and normalised flow patterns. (Raz et al 2022 (53))
Venous sinus stenoses eg transverse sinus (Pereira et al 2021 (54))
Bone dehiscence in sigmoid plate or jugular bulb. (49)(51)
Background on ICH in CFS and Fibromyalgia
Researchers such as Hulens et al 2023 (55)), Wirth et al 2021 (56)), Bragee et al 2020 (57)) and Higgins et al 2017 (51) have been exploring the linking of ICH with Fibromyalgia and Chronic Fatigue Syndrome. Hulens et al 2023 (55)) found that 55 to 85% of ME/CFS patients have symptoms that met the criteria for ICH, and have shown a strong link between idiopathic intracranial hypertension, fibromyalgia, and chronic fatigue syndrome, suggesting they may share a common underlying pathophysiology of increased cerebrospinal pressure.
Higgins et al (58)) describe “recognition of similarities between chronic fatigue syndrome and idiopathic intracranial hypertension (IIH) has raised suggestions that they might be connected, with chronic fatigue syndrome representing a mild version of IIH, sharing many of its symptoms, but without the signature features of elevated intracranial pressure that characterize the complete syndrome. Cranial venous outflow obstruction has been proposed as the pathological substrate.”(58) Wirth et al 2021 (56)) described the accumulating evidence of endothelial dysfunction, muscle and cerebral hypoperfusion in ME/CFS patients with an increase in intracranial pressure.
Higgins et al (58)) found 20% of patients with CFS had high CSF pressures, but more interesting was that 80% felt significantly better after a lumbar puncture, improving headaches, alertness and reducing fatigue, often lasting for weeks. Researchers and clinicians have been looking for spontaneous CSF leaks to explain the variations, but we believe this is not the solution in most, as this represents a simple change from IJV to vertebral venous flow dysfunction as primary cause, compounded by CSF/lymphatic/glymphatic obstruction.
Physiology of ICH
Townsend and Fargen 2021 (59)) describe “Intracranial CSF pressures and intracranial venous pressures are coupled by arachnoid granulations, which exist predominantly in the superior sagittal sinus (SSS). Animal studies have demonstrated that unidirectional flow of CSF from the subarachnoid space into the venous sinuses through these granulations occurs at a pressure gradient of 3–5 mmHg.”
“As intracranial venous pressure rises, the pressure within the subarachnoid space (ICP) will rise until it is 3–5 mmHg higher than the venous sinus, at which point CSF will drain across the arachnoid granulations. This equilibrium is the basis of the connection between intracranial pressure and venous sinus pressure.”(59)
Blood leaves the brain as a result of back propulsion of the residual arterial pressure, complemented by a respiratory mechanism. The latter is the thoracic pump which produces a negative intrathoracic pressure during inspiration increasing the aspiration of blood towards the right atrium. In addition to these, changes in posture and gravity play a main role in ensuring correct cerebral venous return.
Studies have found cerebral blood flow abnormalities and metabolic changes in POTS and Long COVID, even in those whose POTS symptoms have improved.(Wei & Morrison 2023 (60.)) Neuroimaging studies have shown signs of neuroinflammation in Long COVID patients, which could potentially lead to increased intracranial pressure.(54) This can then be linked to the POTS and Long COVID SPECT scan changes, with patterns of brainstem hypoperfusion, and patchy cerebral hyperperfusion with the underlying causes of vascular and autonomic dysfunction, and the Amino Acid dysfunction with secondary aspartic acid/glutamate dysfunction linked to blood-brain barrier disruption.
Similarly some ME/CFS and POTS patients show signs of intracranial hypertension on imaging studies, and symptoms like headaches, cognitive impairment, and visual disturbances in ME/CFS may be related to increased intracranial pressure.(55)
Research from Larsen 2020 (61)) and others eg Townsend et al 2023 (59)) are challenging the traditional theories of Idiopathic Intracranial Hypertension, demonstrating where CSF and lymphatic dysfunction link with venous flow abnormality. The most common cause seen in clinic studies is from venous outflow obstruction, involving the dural venous sinuses, Internal Jugular Veins, and Vertebral veins. Fargen et al (62)) found venous sinus outflow obstruction in 30-90% of IIH patients. Dwyer et al 2013 (63)) showed evidence of venous obstruction in the dominant side circulation in MR venograms in 52% of suspected IIH, ands 17 out of 20 cases with available CSF opening pressure when elevated showed dominant-sided venous outflow obstruction.
While the internal jugular veins are primary pathways for cerebral venous drainage when supine, the vertebral venous system serves as a significant collateral route, especially when jugular outflow is compromised. When supine, the dominant venous outflow is through the IJVs. When erect, it transfers to the vertebral veins. When both systems are compromised, our research points to the emissary veins as the main venous outflow.
ICH is more complex than just CSF and venous obstruction, as it involves obstruction of lymphatic channels, the newly discovered CSF Canalicular system which in turn impact on the glymphatic system. The fascial layers of the head and neck including the carotid sheath, and the effect of upper cervical dysfunction particularly in Ehlers-Danlos Syndrome and after neck trauma further complicate functional disorder.
Fascial changes noted by clinic lymphatic therapists in the fascial planes of the neck, with obstruction of the lymphatics in these areas, notably in the carotid sheath at the base of the neck provide a sound hypothesis for the backpressure from lymphatic obstruction affecting CSF pressure, inducing Intracranial Hypertension (ICH), especially when compounded by venous obstruction in the neck pushing pressure up in the dural sinuses of the brain affecting the CSF pressure required to transfer toxins from the CSF to the dural sinuses via the arachnoid granulations.
This system is often dysfunctional after SARS-CoV infections, studies demonstrating impaired CSF outflow and glymphatic dysfunction.(Wu et al 2023 (64)) (Zhou et al 2024 (65))
The lymphatic and fascial dysfunction has largely been ignored in medicine, but these have been shown to have significant effects on ICH, and I believe, the potential impact of obstruction of the CSF Canalicular System by Joel Pessa 2023 (66)) at C1 where the vessel sits in the vulnerable interstitia of the IJV, or at the base of the neck in the Thoracic Outlet Syndrome.
Overview of Potential Causes of Intracranial Hypertension
The research into the venous obstruction syndromes has shown this to be the commonest cause of ICH, but it does not explain all.
Intracranial hypertension can result from various aetiologies, including venous outflow obstruction. While the internal jugular veins are primary pathways for cerebral venous drainage, the vertebral venous system serves as a significant collateral route, especially when jugular outflow is compromised. Obstruction within the vertebral venous plexus can impede cerebrospinal fluid (CSF) drainage, potentially leading to elevated intracranial pressure.
Table 7: Potential Causes of Intracranial Hypertension
1. Disruption in Cerebrospinal Fluid (CSF) Dynamics
Glymphatic dysfunction, with its postural flow variation, and COVID-associated dysfunction
COVID-19-associated astrocyte damage is believed to disrupt the glymphatic system, reducing interstitial fluid clearance. The end feet of the astrocyte line the perivascular channels of the glymphatic system. This dysfunction contributes to fluid retention within the brain and elevated ICP, exacerbated by inflammation and postural dynamics that influence CSF and interstitial fluid flow.
The resultant failure of the glymphatic system contributes to an increase in intracranial fluid load, which can exacerbate symptoms of ICH. This dysfunction is particularly evident in supine positions where CSF flow dynamics are more passive and vulnerable to disruption.
Impaired Absorption of CSF:
Dysfunction or obstruction in the arachnoid granulations (e.g., due to inflammation, thrombosis, or venous hypertension).
Increased venous sinus pressure reduces the CSF absorption gradient.
Overproduction of CSF:
Rare, but observed in certain conditions like choroid plexus tumours.
Obstruction of CSF and Lymphatic Flow:
Obstruction of the head and neck lymphatic pathways at C1 and base of neck
Structural anomalies such as stenosis of the aqueduct of Sylvius or Chiari malformation.
Chiari Malformations, with downward displacement of cerebellar tonsils obstructs CSF pathways.
Obstruction to CSF Canalicular System (65)
Intracranial mass lesions, such as tumours, haematomas, or abscesses increase intracranial volume and pressure.
Craniospinal Congenital Abnormalities- Syringomyelia or tethered cord syndrome can impair CSF flow.
2. Systemic Contributions
Hypercoagulable States:Conditions like antiphospholipid syndrome or malignancy predispose to venous thrombosis.
Inflammatory and Infectious Causes
Meningitis or Encephalitis- Leads to arachnoid granulation dysfunction and venous obstruction.
Autoimmune Disorders:
Vasculitis affecting cerebral venous drainage.
3. Obstruction of venous outflow:
Venous outflow obstruction (e.g., in the transverse sinuses, jugular veins, or vertebral venous plexus) raises intracranial venous pressure. This reduces the pressure gradient required for cerebrospinal fluid (CSF) absorption by arachnoid granulations, and impairs drainage of blood and CSF from the brain.
Conditions like transverse sinus stenosis, jugular vein compression, or cervical spine abnormalities can directly obstruct venous pathways.
The dural venous sinuses (e.g., transverse sinus, sigmoid sinus) act as conduits for venous blood and CSF absorption. Stenosis or thrombosis of the dural sinuses results in increased resistance to venous outflow, and backpressure that impairs the function of the arachnoid granulations, leading to increased CSF volume and intracranial pressure, which contributes to secondary cerebral oedema.
Venous sinus stenosis is commonly observed in idiopathic intracranial hypertension (IIH) and secondary ICH.
Transverse sinus stenosis is often bilateral and leads to increased venous sinus pressure, directly contributing to ICH.
Compression or collapse- elevated intracranial pressure can compress nearby structures, further narrowing the sinus lumen.
Venous thrombosis in the dural sinuses exacerbates pressure dysregulation, often presenting with acute or chronic ICH.
Stenosis of the transverse or sigmoid sinuses impairs venous drainage and raises venous pressure. While still rare, COVID-induced dural sinus thrombosis is described as 60 times more common than non-COVID.(Takasu et al 2024. (67))
Sinovenous thrombosis leads to direct back-pressure in the CSF system.
Elevated Intrathoracic or Intra-abdominal Pressure, where conditions like obesity or Valsalva manoeuvres raise central venous pressure, reducing venous return from the brain.
“Mechanical and Hydraulic” Influences in ICH and brainstem hypoperfusion.
-linking Intracranial HT with obstructed venous outflow from the brain, dural sinus anomalies/dysfunction and enlarged arachnoid granulations
Glymphatic System -detailed in Glymphatic System (Exelby 2023 (68)
Glymphatic dysfunction, with its postural flow variation, and COVID-associated dysfunction is probably the most important component in ICH, especially in the head pressure experienced at night. It may be associated with HPA axis dysfunction with its effects on cortisol and glucose metabolism. It is a key part in cognitive dysfunction and ICH particularly from COVID damage to astrocytes.
Figure 10. The Glymphatic System

Source: Mogensen et al. The Glymphatic System (en)during Inflammation (69)
The glymphatic system is a system of paravascular channels formed by “end feet” of astroglial cells. It is a crucial waste clearance pathway in the brain, that functions differently based on body position and state of consciousness. Glymphatic dysfunction is associated with optic nerve oedema and visual loss in ICH, likely due to impaired fluid drainage along the optic nerve sheath. (Bezerra et al 2018 (70)), (Jones et al 2021. (71)) The Virchow-Robin spaces (perivascular spaces) play a crucial role in CSF drainage and waste clearance. Impaired function in these spaces has important implications in Alzheimers disease, traumatic brain injury and aging. (Cherian et al 2016 (72)) (Gouveir-Freitas et al 2021 (73))
Its key facts is:
Macroscopic system for waste clearance in brain (brain’s sewer) of solutes such as amyloid-beta, tau, and other metabolites.
Key functions include Interstitial fluid dynamics, facilitating waste clearance from the brain into the venous circulation and CSF-ISF exchange, regulating fluid homeostasis within the CNS.
Waste drains through dural venous sinuses of the brain via Arachnoid Granulations, CSF Canalicular System, deep and superficial lymphatic channels
Glymphatic functioning plays a critical role in intracranial hypertension and cognitive impairment
This process is driven by aquaporin-4 (AQP4) channels on astrocytic end feet surrounding cerebral vasculature.
Glymphatic transport is more efficient when lying down compared to an upright position, characterized by slower clearance and more cerebrospinal fluid (CSF) efflux along larger cervical vessels (Lee et al 2015 (74))
Transport is most efficient when lying on one's side. (Lee et al 2015 (74)) (Reddy & van der Werf 2020 (75))
Glymphatic function is dramatically enhanced during sleep (Reddy & van der Werf 2020 (75)) (Ren et al 2021 (76))
The system is up to 90% more active during sleep compared to wakefulness (Reddy & van der Werf 2020 (75))
Slow-wave sleep (N3 stage) is particularly important for increased glymphatic clearance (Ren et al 2021 (76))
During wakefulness, the glymphatic system remains mainly disengaged (Reddy & van der Werf 2020 (75))
CSF flow has a small-amplitude rhythm during wakefulness, peaking at around 0.25 Hz (Reddy & van der Werf 2020 (75))
Figure 11. CSF Circulation through Glymphatic Channels and drain through Arachnoid Granulations and Meningeal Lymphatics

Source: Shen MD. Cerebrospinal fluid and the early brain development of autism. (77)
Glymphatic Dysfunction Following COVID-19.
COVID-19 infection can lead to glymphatic system dysfunction, with inflammatory responses, blood-brain barrier (BBB) disruption and neurovascular changes. (Zhou et al 2024 (65)) It can cause thromboinflammatory disorders causing arteritis in the vessels, thromboses in the dural sinuses as well as direct damage to the astrocytes and other glial cells, compounding the cognitive impairment from ICH from astrocyte/glymphatic dysfunction.
COVID preferentially infects astrocytes in the brain, causing marked metabolic changes, impairing their ability to fuel neurons and produce neurotransmitters.(98)(99) This increases astrocyte reactivity and cellular stress, altering cellular morphology and branching complexity.(Huang & Fischell 2022 (76)) (Rosu et al 2019 (78)) Damaged astrocytes upregulate pro-inflammatory genes such as SERPINA3 and S100A10 (Colinet 2024 (79)) and release inflammatory mediators that damage other components of the BBB including endothelial cells and pericytes.( Zhang et al 2023 (79))
This evolving understanding now aligns with clinical observations of PEM resolution following lymphatic release, particularly when involving cervical and thoracic flow pathways. These data suggest that glymphatic-lymphatic drainage may constitute a bottleneck in patients with exertional intolerance, and that interventions targeting the fascial-lymphatic axis may offer therapeutic benefit.
The disruption of the glymphatic system reduces interstitial fluid clearance which contributes to fluid retention within the brain and elevated ICP, exacerbated by inflammation and postural dynamics that influence CSF and interstitial fluid flow. This dysfunction is particularly evident in supine positions where CSF flow dynamics are more passive and vulnerable to disruption. (Rosu et al 2019 (80))
Astrocyte end feet are damaged affecting their role in maintaining the BBB. Loss of AQP4 polarity with AQP4 expression spreading from end feet to cover the entire astrocyte, educed end feet coverage of blood vessels (Rosu et al 2019 (80)) discontinuous perivascular end feet layers with oedema (Zhang et al 2023 (81)) and compromised interaction between astrocytes and vascular basement membranes (Rosu et al 2019 (80))
The reduction in end feet, decreased AQP4, changes to basement membranes, loss of endothelial tight junction suggest changes to the blood brain barrier (BBB) can be linked to inflammatory signalling and hypoxia. (80) This results in a reduced ability to transport physiological molecules and remove interstitial waste from the brain. Decreased glymphatic activity potentially contributing to cognitive impairment, while the widespread astrogliosis in the brain leads to adverse effects on other cell populations. (Colinet 2024 (79))
COVID research has demonstrated:
Bilateral Asymmetric Decline: Recovered mild COVID-19 patients showed asymmetric bilateral glymphatic function decline after four months of recovery (Wu et al 2023 (82))
Age-Related Impact: The decrease in glymphatic function was more pronounced in older recovered patients, (82) in keeping with the known association in glymphatic mal-functioning in Alzheimer’s disease. (Cherian et al 2016 (72))(Gouvela-Freitas & Bastos-Leite 2021 (73)) In a study of over 6 million people over 65 years, COVID increased Alzheimer’s risk by 50 to 80% within a year most noticeable in women over 85. (Hahn 2022 (83))
Cognitive Correlation: Patients with right-sided glymphatic dysfunction experienced a higher proportion of cognitive decline compared to those with left-sided dysfunction (82)
CSF Clearance Impairment: Long COVID patients showed reduced cerebrospinal fluid clearance, suggesting potential glymphatic system congestion (Zhou et al 2024 (84))
Venous Obstruction
Venous obstruction directly increases venous sinus pressure, impairing CSF absorption and leading to a compensatory increase in CSF production or brain oedema. Prolonged venous hypertension further exacerbates fluid dynamics, resulting in ICH.
a. Cervical Vertebral Venous Flow Obstruction and Retrograde Flow
Vertebral venous obstruction and retrograde flow (described above) are observed clinically and exacerbate venous hypertension in the craniocervical junction. This dynamic, particularly in the supine position, increases intracranial venous congestion and aggravates ICH symptoms.
Studies have highlighted the compensatory role of the vertebral venous plexus in conditions like bilateral transverse sinus stenosis, where the vertebral venous plexus may enlarge to accommodate increased venous pressure, thereby mitigating symptoms of intracranial hypertension. If this compensatory mechanism is overwhelmed or if the vertebral venous plexus itself becomes obstructed, intracranial pressure can rise, exacerbating ICH symptoms. (Hepworth et al 2021 (85))
Obstruction within the vertebral venous plexus can impede cerebrospinal fluid (CSF) drainage, as well as meningeal lymphatic drainage into the deep lymphatic system, potentially leading to elevated intracranial pressure. This compromised drainage can elevate intracranial venous pressure, subsequently increasing CSF pressure leading to ICH.
Cervical spine instability, hypermobility and trauma have been implicated in such venous obstructions. Structural abnormalities in the cervical region may compress venous pathways, including the vertebral venous plexus, hindering effective cerebral venous outflow.
Figure 12. Summary of the positional changes in craniocervical venous structure between supine and upright posture.

Source: Kosugi,K et al Posture-Induced Changes in the Vessels of the Head and Neck: Evaluation using conventional Supine CT and Upright CT. Nature (Scientific reports) 2020. https://www.nature.com/articles/s41598-020-73658-0 (86)
b. Internal Jugular Vein (IJV) Obstruction at C1
Obstructions of the IJVs at the C1 level impede venous outflow, particularly in the supine position when cerebral venous outflow is primarily through the IJVs. This readily seen in the Spectral CT venography where obstruction can be demonstrated between the transverse process of C1 and the stylohyoid. Dynamic ultrasound of the IJVs in clinic scanning solidifies the IJV dysfunction with valves within the IJV frequently dysfunctional or stenosed.
This can lead to venous stasis, retrograde flow, and redirection of venous drainage to the vertebral venous system, where ICH can occur if there is from vertebral venous flow abnormality eg from Nutcracker or Pelvic Congestion Syndrome.
Postural changes may amplify these dynamics, increasing cranial venous pressure and ICP. This is frequently linked to C1 vertebral instability and hypermobility (and often associated with malrotation in the upper cervical vertebrae, allowing significant improvements in many with attention to posture and neck.
The severity of symptoms of head pressure with postural change may guide clinicians to the underlying causes. Supine pressure is likely to involve IJV obstruction at C1 or base of neck usually with associated Thoracic Outlet Syndrome (TOS,) both magnified by vertebral venous obstruction (from neck or Nutcracker/Pelvic Congestion)
As described above, erect head pressure appears to be more associated with cervical vertebral venous obstruction, and when retrograde flow is present, usually both. Research into defining these patterns more clearly is continuing.
c. Dilatation of the Carotid Sheath- IJV Obstruction at Base of Neck
The IJV obstruction at the base of the neck is less-clearly defined. These appear to be associated with changes to the tissues in the veins themselves, as arterial TOS surgery does not necessarily remove the IJV obstruction. ATOS surgery primarily targets arterial compression relief through thoracic outlet decompression (TOD), including first rib resection, scalenectomy, and neurolysis/arteriolysis. While these procedures address subclavian artery compression, they do not typically involve direct intervention on the IJV, which lies outside the primary surgical field for ATOS. (Teijink et al 2023 (87)) (de Kleijn et al 2023 (88))
There is little available literature to address this concern, leaving a stenosed IJV at the base of the neck as an unresolved problem in many patients. Botulinum toxin (botox) has been used to target the scalene muscles to relieve neurovascular compression,(89)(90) and IJV valve repair/stenting is currently at an experimental stage,(Zhou et al 2018 (91)) leaving physical therapy as the current best option, with attention to associated lymphatic obstruction to help relieve the ICH that may have developed.
Figure 13. IJV Obstruction between Stylohyoid and C1 transverse process

Courtesy: Dr Zane Sherif, Mermaid Beach Radiology
Figure 14. Examples of Dilated IJVs at base of neck

Courtesy: Dr Zane Sherif, Mermaid Beach Radiology
Figure 15. Effect on IJV Obstruction from ATOS Surgery
This demonstrates the ineffectiveness of current surgical techniques on IJV obstruction at the base of the neck.

Courtesy: Dr Zane Sherif, Mermaid Beach Radiology
The carotid sheath contains critical structures like the common carotid artery, vagus nerve, and lymph nodes. Although the carotid sheath dilatation can be readily seen on CT venography, there is little formal research into the base of neck obstruction directly examining the relationship between internal jugular vein (IJV) dilatation (from valve stenosis or thoracic outlet syndrome) and carotid baroreceptor dysfunction, although several studies provide relevant insights into potential mechanistic connections between these conditions.
The carotid baroreceptors and IJV share intimate anatomical proximity within the carotid sheath. Baroreceptors, which are stretch receptors located at the bifurcation of external and internal carotid arteries in the carotid bulb, are innervated by the glossopharyngeal nerve. This anatomical arrangement creates potential for mechanical interactions when one structure becomes pathologically altered. (Suh et al 2012 (92))
Descriptions of symptoms from obstruction are gleaned from IJV thrombosis by Scerrati et al 2021 (93)) with headaches, pulsatile tinnitus, blurred vision, diplopia, and cognitive fog due to intracranial venous hypertension.
These symptoms potentially overlap with manifestations of baroreflex dysfunction, which include "volatile hypertension with periods of hypotension" and "orthostatic tachycardia or orthostatic intolerance.” The baroreflex normally serves to buffer blood pressure against excessive rise or fall. When impaired, there is a “loss of buffering ability, and wide excursions of pressure and heart rate occur." (Ketch et al 2002 (94))
d. Vertebral Venous Flow Dysfunction (Nutcracker and Pelvic Congestion Syndromes)
Nutcracker syndrome and pelvic congestion can contribute to vertebral venous congestion and intracranial hypertension through shared mechanisms of venous outflow obstruction and collateral pathway activation.
Figure 16. Enlarged Paraspinal Veins and Paravertebral Varix

Source: Dr Zane Sherif Mermaid Beach Radiology
Left renal vein compression between the aorta and superior mesenteric artery elevates renal venous pressure, forcing blood into collateral pathways like the lumbar veins and vertebral venous plexus (VVP.) Retrograde flow into the vertebral venous plexus increases epidural venous congestion, which may impair cerebrospinal fluid (CSF) absorption and elevate intracranial pressure (ICP.) (Scholbach 2017 (95))
Pelvic venous incompetence (e.g., ovarian vein reflux) causes pelvic venous hypertension, redirecting blood to the ilio-vertebral venous network. (Bałabuszek et al 2022(96)). Chronic pelvic venous stasis may propagate retrograde pressure to the VVP, exacerbating cerebral venous congestion and ICP. (Arun et al 2022 (97)) (Tuță 2022 (98))
In bilateral transverse sinus stenosis, VVP dilation compensates for impaired jugular drainage, temporarily mitigating ICP elevation. (Li et al 2022 (98) Elevated VVP pressure reduces the pressure gradient between cerebral veins and the right atrium, impairing venous outflow. (Arun et al 2022 (96)) This may trigger a "Starling resistor" effect, where CSF absorption declines as venous pressure rises, creating a feedback loop that sustains high ICP. (Arun et al 2022 (96)) (Tuță 2022 (97))
Case studies report headaches resolving after lumbar vein embolization, reducing vertebral venous plexus congestion. (Devcic et al 2022 (99)) (Scholbach 2017 (95))
The absence of valves in the cranio-vertebral venous system means vena cava pressure directly reflects in CSF pressure, making venous outflow obstacles (even those located in cervical, thoracic, or abdominal regions) particularly impactful for intracranial pressure.(Tuta 2022 (97))
Obstruction of the CSF Canalicular System (C1 and Base of Neck)
The implications of the discovery of the CSF canalicular System by Joel Pessa (66) suggests its obstruction may be a primary factor in the development of ICH. It functions through gravitational influences, and it a likely cause of increasing head pressure when erect, particularly in the absence of venous outflow obstruction. It may represent the primary route for CSF outflow from the cerebral system. At present this only exists in anatomical specimens with no functional studies. Its potential obstruction at C1 and base of the neck places it at potential risk for CSF obstruction and secondary ICH.
In keeping with the evolving hypothesis of posture-related obstruction of the IJVs, we believe postural changes affecting cervical spine alignment may worsen this obstruction, especially in the erect position. This may be complicated by the fascial changes clinic studies have seen in the C1 region. Given the complexity of ICH/ head pressure when erect this is an area that requires considerable attention..
The potential for increased pressure from CSF Canalicular obstruction is magnified when there is vertebral venous obstruction
.
Meningeal Lymphatic Obstruction
Meningeal lymphatic vessels run down toward the base of the skull along the sinus, the vein, and the meningeal arteries and drain out of the skull via the foramina of the base of the skull alongside arteries, veins, and cranial nerves.
Cerebrospinal fluid (CSF) enters the parenchyma by bulk flow along paravascular spaces,
and ISF is cleared along paravenous drainage pathways. Meningeal lymphatic vessels absorb CSF from the adjacent subarachnoid space and ISF from the glymphatic system and transport fluid into deep cervical lymph nodes via foramina at the base of the skull.
The meningeal lymphatic system plays a crucial role in the clearance of waste products from the brain, and its obstruction can contribute to raised intracranial pressure. At the foramen magnum, this system may be impeded by structural abnormalities, vascular compression, or inflammatory processes, which can further impair the drainage of interstitial fluid and waste products.
In conditions like Chiari I malformation, and, we believe the “plug in the drain” phenomenon, reduced cross-sectional area at the foramen magnum prevents normal dampening of intracranial pressure pulse waves, creating a high-pressure differential across this region. (Heiss 2023 (9))
Meningeal lymphatic dysfunction contributes to multiple pathological conditions related to intracranial pressure. When researchers ablated or blocked meningeal lymphatic vessels in animal models, this intervention impeded hematoma clearance following intracerebral haemorrhage, (Tsai et al 2022 (100)) exacerbated hydrocephalus after intraventricular haemorrhage,(Zhang et al 2024 (101)) and reduced drainage of cerebrospinal fluid components to deep cervical lymph nodes. (Zhang et al 2024 (101))
There are several mechanisms by which lymphatic obstruction at the foramen magnum contributes to intracranial hypertension.
Impaired waste clearance- obstruction prevents efficient removal of metabolic byproducts and CSF, leading to accumulation of potentially neurotoxic substances. (Tuță 2022 (98)) (Zhang et al 2024 (101))
Neutrophil Extracellular Traps (NETS)- contributing to acute lymphatic endothelial cell injury and lymphatic thrombosis, which leads to meningeal lymphatic vessel dysfunction and exacerbates hydrocephalus and brain injury (Zhang et al 2024 (101))
Glymphatic System Dysfunction- patients with idiopathic intracranial hypertension have excess cerebral interstitial fluid and CSF in subarachnoid spaces, suggesting congestion of the glymphatic system (Tuță 2022 (98))
Pressure Gradients- the Starling resistor effect may create a feedback loop where increased pressure further impairs drainage, as pressure gradients between various compartments become disrupted.
In idiopathic intracranial hypertension (IIH), chronic, subtle increases in venous pressure and decreased CSF absorption lead to compensatory phenomena including remodelling of brain parenchyma and shifts in CSF distribution.(Mangalore et al 2019 (102)) Enlarged foramina due to remodelling are noted in IIH, which may represent compensatory mechanisms to improve drainage.(103) Enlarged meningeal lymphatic flow correlates with disease severity in several neurological disorders, suggesting its importance in maintaining normal intracranial pressure.(103)
Lymphatic Obstruction and Autonomic connection
Lymphatic obstruction in the head and neck is often seen clinically. It is almost to impossible to confirm radiologically, although large nodes may be seen eg in the cervical collar, or periodically seen in IJV dynamic ultrasounds. The anatomy of the arteries and veins in the head and neck is such that the lymphatics envelop the vessels, so any physical compression of the vascular structure must result in varying degrees of lymphatic obstruction.
The lymphatic system, including dural lymphatics, plays a role in CSF drainage from the brain to systemic circulation, and is particularly important when venous outflow is obstructed.. Dysfunction in the lymphatic CSF pathways exacerbate intracranial pressure.
Jacob, Boisserand et al 2019 (104) confirmed in mice studies that “vertebral lymph vessels connect to peripheral sensory and sympathetic ganglia and form similar vertebral circuits connecting to lymph nodes and the thoracic duct. They showed that the connection between lymph vessels and sympathetic ganglia occurred at the surface of the ganglia revealing a hitherto unknown anatomical interaction between the autonomous nervous system and vertebral lymphatic vessels. They are closely apposed around the chains of sensory and sympathetic nervous ganglia, so lymphatic vessels may provide molecular signals to the sympathetic neurons that control vascular tone of lymphatic ducts and cerebral arteries and arterioles.”
“Previous observations by the authors also showed that adrenergic fibres connect to the thoracic lymphatic duct and also innervate the wall of lymph node arterioles. The crosstalk between spine LVs and the sympathetic system is thus likely relevant for the regulation of peripheral lymph and glymphatic drainage and may coordinate them with the activity of brain and spine tissues. The authors speculate that a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage.”(104)
Albayram et al 2022 (105) showed “dural lymphatic structures along the dural venous sinuses in dorsal regions and along cranial nerves in the ventral regions in the human brain and they detected direct connections between lymphatic fluid channels along the cranial nerves and vascular structures and the cervical lymph nodes. They also identified age-related cervical lymph node atrophy and thickening of lymphatics channels in both dorsal and ventral regions, findings which reflect the reduced lymphatic output of the aged brain.”(105)
“Macromolecules, waste products, and excess fluid from most tissues are known to drain into the systemic lymphatic system. Classically, absorption of CSF occurred through arachnoid granulations and villi of the intracranial and spinal venous sinuses. More recent animal studies have demonstrated CSF-ISF drainage via meningeal lymphatic vessels and along the cranial nerves into deep cervical lymph nodes.” According to their study result, the vascular-carotid space in the neck is very important for the CSF-ISF drainage from the brain.”(105)
The discovery of this inter-relationship between vertebral lymph vessels and sympathetic ganglia provides a tantalizing possibility to help explain reduced flow in the middle cerebral artery and brainstem – from the increased head pressure at the jugular foramen and the increased venous pressure at the crowded craniocervical junction as the posture changes from lying to standing, the venous return changes from Internal Jugular to Vertebral venous plexus. If the IJV is obstructed, for example by poor head-forward posture, the venous pressure in the vertebral system is increased further, and the lymphatics too are increasingly compressed, and theoretically the activation of the sympathetics in the lymphatic walls.
These lymphatic vessels have no valves, so sustained obstruction will cause backpressure into the Glymphatic system with the risk of Idiopathic Intracranial Hypertension.
Deep Lymphatic Flow Obstruction in the Carotid Sheath
Deep lymphatic flow obstruction within the carotid sheath hinders drainage from the brainstem and upper cervical structures, resulting in fluid accumulation. Postural changes can worsen lymphatic drainage, especially when the cervical region is compressed or realigned.
The deep cervical lymphatic flow, particularly within the carotid sheath, is critical for the drainage of fluid from the brainstem and upper cervical region. Obstruction of this lymphatic pathway can contribute to elevated ICP by impairing the removal of excess fluid and waste products from the deep structures of the brain, and which in many POTS patients with ICH both vertebral and deep cervical lymphatic obstruction coexists.
From the work by Jacob, Boisserand and Albayram et al (104)(105), it is a logical extension to apply these to the hypoperfusion through sympathetic activation from the obstructed lymphatics as well as direct postural effects on blood flow to the structures in the head and neck and cerebral tissues. The drainage from the CSF canalicular system is to the subclavian veins, and is only active in an upright position, making the venous Thoracic Outlet Syndrome potentially far more important than previously recognized. The primary difficulty in assessing this CSF canalicular system is that there have been no live dynamic studies available.
Lymphatic Obstruction and Fascial Changes head and neck
Lymphatic obstruction in the head and neck region triggers complex cascades of fascial and soft tissue changes that progress from initial inflammation to eventual fibrosis. Slater et al 2024 (106)) describe fascia as being highly innervated, rich with blood vessels, lymphatics and hormonal and neurotransmitter receptors, and a regulatory system that can both exacerbate inflammation and provide therapeutic targets for intervention. Their research suggests that fascial tissues play a critical role in inflammatory cascades, where "facial regulation of inflammation can provide an efficient therapeutic target to counteract chronic pain-inflammation-stress cascades."
Following head and neck tissue injury (such as from cancer treatment, and a result of hypoxia-driven RAGE activation), pro-inflammatory cytokines—particularly IL-1β, IL-6, and TNF-α—trigger a cascade of events leading to fascial pathology:
These cytokines deactivate the local lymphatic pump mechanism
The fibroblasts differentiate into myofibroblasts
TGF-b1 released by these cells causes contraction of fascial tissues
Which compresses pre-lymphatic pathways
The combined effects create a pathological feed-forward loop leading to "interstitial inflammatory stasis.” (Tuckey et al 2021 (107))
Lymphatic obstruction leads to predictable stages of fascial change:
Early changes- palpable thickening and tightness of the dermis without visible swelling
Intermediate stage- visible tissue swelling that transitions from soft and reducible to firm and non-reducible
Advanced stage- development of extreme hardness with "woody texture"
Severe fibrosis- characterized by soft tissue contracture.
Shear wave elastography (SWE) demonstrates dramatic increases in tissue stiffness in affected areas, with measurements revealing substantial decreases in soft tissue elasticity compared to normal tissues. In one case study, SWE speeds in fibrotic neck tissues were "dramatically higher than those reported in other normal soft tissues or pathologic states." (Deng et al 2018 (108))
Chiari Malformations
Chiari malformations involve structural displacement of the cerebellar tonsils through the foramen magnum, leading to impaired CSF flow and venous congestion.
In the supine position, this malformation can exacerbate CSF accumulation due to gravitational redistribution, contributing to hydrocephalus-like symptoms and elevated ICP.
Postural changes may increase symptom severity due to further compression of CSF pathways at the craniocervical junction.
Enlarged Arachnoid Granulations (COVID-19 Association)
Arachnoid granulations are projections of the arachnoid membrane into the dural venous sinuses, facilitating cerebrospinal fluid (CSF) absorption into the venous system. They are commonly located within the transverse sinuses, superior sagittal sinus, and parasagittal venous lacunae, usually ranging from 2 to 8 mm in size. However, they can occasionally exceed 10 mm, with some studies reporting sizes up to 15–20 mm. Their size and number typically increase with age, and they are present in approximately two-thirds of individuals.
Arachnoid granulations act as passive, pressure-dependent valves, and play a crucial role in CSF drainage into the venous system.. Enlargement of arachnoid granulations is frequently associated with venous hypertension as increased dural sinus pressure causes compensatory hypertrophy of arachnoid granulations as they attempt to accommodate the elevated pressure. Reduced efficiency of CSF drainage into the venous system leads to compensatory granulation hypertrophy.
Enlarged arachnoid granulations, frequently noted in post-COVID patients in Long COVID investigations, can obstruct CSF absorption in the venous sinuses which may lead to fluid accumulation and increased ICP, particularly in cases where compensatory venous drainage mechanisms are already impaired. There appears to be however, no direct studies on the effect of COVID on these granulations.
These granulations may also contribute to disrupted CSF resorption in the erect posture due to altered venous sinus dynamics. Enlarged granulations may paradoxically obstruct dural sinuses, exacerbating venous hypertension and further impairing CSF absorption.
Results of Clinic Investigations
The major contributing causes found in clinic investigations in Intracranial Hypertension include:
1. Glymphatic dysfunction, especially after COVID, probably the most important component in ICH, especially in the head pressure experienced at night. It is associated with HPA axis dysfunction with its effects on cortisol and glucose metabolism.
2. Internal Jugular vein Obstruction at the C1 region
3. Internal Jugular vein Obstruction at based of neck associated with Thoracic Outlet Syndrome
4. IJV valve dysfunction / fibrosis/stenosis, cause thought to be associated with the C1 obstruction
5. Cervical vertebral venous obstruction
6. Vertebral venous obstruction- Nutcracker and Pelvic Congestion Syndromes
7. Retrograde cervical vertebral venous flow (“plug in the drain”)
8. Lymphatic obstruction at craniocervical junction
9. Lymphatic obstruction in carotid sheath at C1 between the stylohyoid and transverse process of C1
10. Lymphatic obstruction at base of neck, which may be reflected in widespread lymphatic obstruction
Conclusion
This document proposes a paradigm shift in the understanding of intracranial hypertension (ICH) in POTS, ME/CFS, Long COVID, and fibromyalgia, reframing it as a dynamic, posture-sensitive, and hydraulically integrated disorder. The pathogenesis of intracranial hypertension in POTS and allied syndromes (ME/CFS, Long COVID, fibromyalgia) is far more complex than previously assumed.
Mechanistic insights drawn from dynamic imaging, anatomical correlations, and clinical symptomology reveal that venous outflow obstruction—particularly at the vertebral and internal jugular levels—is central to this process. These obstructions, often positional and compounded by fascial tension, thoracic outlet dysfunction, and connective tissue laxity (e.g., Ehlers-Danlos syndrome), impair not only cerebral venous drainage but also CSF flow and lymphatic outflow. The result is a fluctuating pressure regime that manifests as orthostatic head pressure, cognitive dysfunction, pulsatile tinnitus, and visual disturbances—hallmarks of ICH in this patient population.
Clinic-based imaging data—using Spectral CT arteriography and venography in a single rapid scan, MRI brain venography, and dynamic ultrasonography—have unmasked key phenomena such as retrograde vertebral venous flow, cerebellar tonsillar descent, and emissary vein overload, consistent with a "plug-in-the-drain" model of upright intracranial hypertension, while introducing the impact of the valveless vertebral venous system overwhelmed by Nutcracker and Pelvic Congestion Syndromes. When compounded by fascial changes, thoracic outlet obstruction, and EDS-related tissue laxity, these mechanical factors create a vulnerable hydrostatic system subject to rapid perturbation.
Importantly, the integration of glymphatic and meningeal lymphatic flow into the ICH model broadens the clinical relevance of these findings beyond neurology into systemic inflammatory and metabolic realms. The evidence strongly supports that cognitive dysfunction ("brain fog"), orthostatic headaches, pulsatile tinnitus, and visual disturbances are not isolated phenomena but reflect the downstream impact of these interlinked hydraulic disturbances.
The resolution of PEM with lymphatic therapy challenges traditional models and supports a revised framework where impaired metabolic clearance, not just mitochondrial insufficiency, underpins exertional collapse. This adds weight to the broader hypothesis of hydraulic, immune, and metabolic obstruction at the craniocervical and systemic levels in dysautonomia.
Therapeutically, emphasis must shift from symptomatic control toward restoration of flow—venous, CSF, and lymphatic—via targeted physical therapy, postural correction, fascial decompression, and selective interventional approaches. This hydraulically informed model provides a robust and scalable framework through which the overlapping pathophysiology of POTS, ME/CFS, Long COVID, and fibromyalgia can be better understood, diagnosed, and treated.
Future diagnostic protocols must include upright assessments of venous and lymphatic drainage, cervical biomechanics, and postural dynamics. Therapeutic avenues targeting CSF-lymphatic drainage, fascial decompression, and venous outflow restoration offer promising interventions. This refined understanding provides a comprehensive framework for addressing the true root causes of postural neurovascular symptoms in these complex patients, moving beyond symptomatic control toward mechanistic resolution.
References:
1. History of POTS with Phillip Low, MD. The Dysautonomia Project. 2020. https://thedysautonomiaproject.org/history-of-pots-with-phillip-low-md/
2. Exelby, G. Brainstem Hypoperfusion in POTS, CFS, Fibromyalgia, Long COVID and GWS. MCMC Research. 2025 https://www.mcmc-research.com/post/brainstem-hypoperfusion-in-pots-cfs-fibromyalgia-long-covid-and-gws-a-key-role
3. Exelby, G. Brain SPECT changes in POTS, CFS, FMS and Long COVID. MCMC-Research. 2025. https://www.mcmc-research.com/post/brain-spect-changes-in-pots-cfs-fms-and-long-covid
4. Hulens M, Dankaerts W, Rasschaert R, Bruyninckx F, De Mulder P, Bervoets C. The Link Between Empty Sella Syndrome, Fibromyalgia, and Chronic Fatigue Syndrome: The Role of Increased Cerebrospinal Fluid Pressure. J Pain Res. 2023;16:205-219https://doi.org/10.2147/JPR.S394321
5. Ray JC, Pham X, Foster E, Cheema S, Corcoran SJ, Matharu MS, Hutton EJ. The prevalence of headache disorders in Postural Tachycardia Syndrome: A systematic review and meta-analysis of the literature. Cephalalgia. 2022 Oct;42(11-12):1274-1287. doi: 10.1177/03331024221095153. Epub 2022 Apr 25. PMID: 35469447.
6. Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, Garland EM, Gamboa A, Diedrich A, Raj V, Sheldon RS, Biaggioni I, Robertson D, Raj SR. The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J Intern Med. 2019 Oct;286(4):438-448. doi: 10.1111/joim.12895. Epub 2019 Apr 16. PMID: 30861229; PMCID: PMC6790699.
7. Ganesh R, Munipalli B. Long COVID and hypermobility spectrum disorders have shared pathophysiology. Front Neurol. 2024 Sep 5;15:1455498. doi: 10.3389/fneur.2024.1455498. PMID: 39301475; PMCID: PMC11410636
8. Yousry I, Förderreuther S, Moriggl B, Holtmannspötter M, Naidich TP, Straube A, Yousry TA. Cervical MR imaging in postural headache: MR signs and pathophysiological implications. AJNR Am J Neuroradiol. 2001 Aug;22(7):1239-50. PMID: 11498410; PMCID: PMC7975193.
9. Heiss JD. Cerebrospinal Fluid Hydrodynamics in Chiari I Malformation and Syringomyelia: Modeling Pathophysiology. Neurosurg Clin N Am. 2023;34(1):81-90. doi:10.1016/j.nec.2022.08.007
10. Muccio M, Sun Z, Chu D, Damadian BE, Minkoff L, Bonanni L, Ge Y. The impact of body position on neurofluid dynamics: present insights and advancements in imaging. Front Aging Neurosci. 2024 Nov 8;16:1454282. doi: 10.3389/fnagi.2024.1454282. PMID: 39582951; PMCID: PMC11582045.
11. Williams H. A unifying hypothesis for hydrocephalus, Chiari malformation, syringomyelia, anencephaly and spina bifida. Cerebrospinal Fluid Res. 2008;5:7. Published 2008 Apr 11. doi:10.1186/1743-8454-5-7
12. Lee RK, Griffith JF, Leung JH, Chu WC, Lam TP, Ng BK, Cheng JC. Effect of upright position on tonsillar level in adolescent idiopathic scoliosis. Eur Radiol. 2015 Aug;25(8):2397-402. doi: 10.1007/s00330-015-3597-3. Epub 2015 Mar 20. PMID: 25791638.
13. Bancroft,M et al. Intracranial pressure and pulsatility in different head and body positions. Brain Communications 2025. https://doi.org/10.1093/braincomms/fcaf115
14. Spiessberger A, Dietz N, Gruter B, Virojanapa J. Ehlers-Danlos syndrome-associated craniocervical instability with cervicomedullary syndrome: Comparing outcome of craniocervical fusion with occipital bone versus occipital condyle fixation. J Craniovertebr Junction Spine. 2020 Oct-Dec;11(4):287-292. doi: 10.4103/jcvjs.JCVJS_166_20. Epub 2020 Nov 26. PMID: 33824558; PMCID: PMC8019109.
15. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010 Aug;28(3):593-617. doi: 10.1016/j.ncl.2010.03.003. PMID: 20637991; PMCID: PMC2908600.
16. Vadera, S. Idiopathic Intracranial Hypertension. 2023 Radiopedia. https://radiopaedia.org/articles/idiopathic-intracranial-hypertension-1
17. Scoffings,D. Intracranial Hypotension. 2023. Radiopedia. https://radiopaedia.org/articles/intracranial-hypotension-1
18. Cerebrospinal Fluid Leak. Cedars Sinai. 2022. https://www.cedars-sinai.org/health-library/diseases-and-conditions/c/cerebrospinal-fluid-leak.html
19. Vemuri NV, Karanam LSP, Manchikanti V, Dandamudi S, Puvvada SK, Vemuri VK. Imaging review of cerebrospinal fluid leaks. Indian J Radiol Imaging. 2017 Oct-Dec;27(4):441-446. doi: 10.4103/ijri.IJRI_380_16. PMID: 29379240; PMCID: PMC5761172.
20. Townsend RK, Fargen KM. Intracranial Venous Hypertension and Venous Sinus Stenting in the Modern Management of Idiopathic Intracranial Hypertension. Life (Basel). 2021 May 31;11(6):508. doi: 10.3390/life11060508. PMID: 34073077; PMCID: PMC8227267.
21. Zhou D, Ding J, Asmaro K, Pan L, Ya J, Yang Q, Fan C, Ding Y, Ji X, Meng R. Clinical Characteristics and Neuroimaging Findings in Internal Jugular Venous Outflow Disturbance. Thromb Haemost. 2019 Feb;119(2):308-318. doi: 10.1055/s-0038-1676815. Epub 2019 Jan 3. PMID: 30605919.
22. Li, M., Sun, Y., Chan, C.C. et al. Internal jugular vein stenosis associated with elongated styloid process: five case reports and literature review. BMC Neurol 19, 112 (2019). https://doi.org/10.1186/s12883-019-1344-0
23. Cejas,C., Cisneros, L.,Lagos,R, Zuk,C, Ameriso,S. Internal Jugular Vein Valve Incompetence Is Highly Prevalent in Transient Global Amnesia. 2010 Stroke. https://doi.org/10.1161/STROKEAHA.109.566315
24. Spinal CSF Leak Foundation. https://spinalcsfleak.org/
25. Iencean SM, Ciurea AV. Intracranial hypertension: classification and patterns of evolution. J Med Life. 2008 Apr-Jun;1(2):101-7. PMID: 20108456; PMCID: PMC3018963.
26. Macey PM. Altered Resting Cerebral Blood Flow in Obstructive Sleep Apnea: A Helpful Change or Not? Sleep. 2015 Sep 1;38(9):1345-7. doi: 10.5665/sleep.4962. PMID: 26285008; PMCID: PMC4531400.
27. Bakalarz M, Rożniecki JJ, Stasiołek M. Clinical Characteristics of Patients with Vertebral Artery Hypoplasia. Int J Environ Res Public Health. 2022 Jul 29;19(15):9317. doi: 10.3390/ijerph19159317. PMID: 35954673; PMCID: PMC9368006.
28. Gokey,R, Das,J. Venous Sinus Thrombosis. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK560598/
29. Letchuman,V, Donohoe,C. Neuroanatomy, Superior Saggital Sinus. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK546615/#:~:text=Symptoms%20related%20to%20superior%20sagittal,loss%2Fhemiparesis%2C%20and%20seizures.
30. Yoshida K, Tsutsumi S, Inami K, Sugiyama N, Ueno H, Ishii H. Large arachnoid granulation protruding into the transverse sinus: A probable cause of intermittent otologic symptoms. Radiol Case Rep. 2023;18(10):3421-3424. Published 2023 Jul 22. doi:10.1016/j.radcr.2023.07.004
31. Huda F, Abdelmonem A, Dehghani Firouzabadi F, Srinivas Dola VN, Sheikhy A, Taheri MR. The role of arachnoid granulations in idiopathic intracranial hypertension. Neuroradiol J. 2023 Dec;36(6):651-656. doi: 10.1177/19714009231173109. Epub 2023 Apr 27. PMID: 37102274; PMCID: PMC10649532.
32. Lu CX, Du Y, Xu XX, et al. Multiple occipital defects caused by arachnoid granulations: Emphasis on T2 mapping. World J Radiol. 2012;4(7):341-344. doi:10.4329/wjr.v4.i7.341
33. Walizai, T (Ed) Aberrant Arachnoid Granulations. Radiopedia. 2024. https://radiopaedia.org/articles/aberrant-arachnoid-granulations
34. VandeVyver V, Lemmerling M, De Foer B, Casselman J, Verstraete K. Arachnoid granulations of the posterior temporal bone wall: imaging appearance and differential diagnosis. AJNR Am J Neuroradiol. 2007 Apr;28(4):610-2. PMID: 17416806; PMCID: PMC7977354.
35. Chapman MN, Fujita A, Sung EK, Siegel C, Nadgir RN, Saito N, Sakai O. Sarcoidosis in the Head and Neck: An Illustrative Review of Clinical Presentations and Imaging Findings. AJR Am J Roentgenol. 2017 Jan;208(1):66-75. doi: 10.2214/AJR.16.16058. Epub 2016 Sep 22. PMID: 27657552.
36. Xu JQ, Liu QQ, Huang SY, et al. The lymphatic system: a therapeutic target for central nervous system disorders. Neural Regen Res. 2023;18(6):1249-1256. doi:10.4103/1673-5374.355741
37. Noé FM, Marchi N. Central nervous system lymphatic unit, immunity, and epilepsy: Is there a link?. Epilepsia Open. 2019;4(1):30-39. Published 2019 Feb 14. doi:10.1002/epi4.12302
38. Hogan BM, Bower NI. Lymphatics and the Brain: It's Time to Go Fishing. Circ Res. 2021 Jan 8;128(1):59-61. doi: 10.1161/CIRCRESAHA.120.318496. Epub 2021 Jan 7. PMID: 33411628.
39. Yoshida K, Tsutsumi S, Inami K, Sugiyama N, Ueno H, Ishii H. Large arachnoid granulation protruding into the transverse sinus: A probable cause of intermittent otologic symptoms. Radiol Case Rep. 2023;18(10):3421-3424. Published 2023 Jul 22. doi:10.1016/j.radcr.2023.07.004
40. Huda F, Abdelmonem A, Dehghani Firouzabadi F, Srinivas Dola VN, Sheikhy A, Taheri MR. The role of arachnoid granulations in idiopathic intracranial hypertension. Neuroradiol J. 2023 Dec;36(6):651-656. doi: 10.1177/19714009231173109. Epub 2023 Apr 27. PMID: 37102274; PMCID: PMC10649532.
41. Dinkin MJ, Patsalides A. Venous Sinus Stenting in Idiopathic Intracranial Hypertension: Results of a Prospective Trial. J Neuroophthalmol. 2017 Jun;37(2):113-121. doi: 10.1097/WNO.0000000000000426. PMID: 27556959.
42. Skorin, L. Determine cause of transient visual loss with thorough questioning. 2005. Primary care Optometry News. https://www.healio.com/news/optometry/20120225/determine-cause-of-transient-visual-loss-with-thorough-questioning#:~:text=Transient%20visual%20obscurations,-The%20final%20major&text=These%20are%20grey%2Douts%2C%20black,often%20aggravated%20by%20postural%20changes.
43. Idiopathic Intracranial Hypertension. National Eye Institute. 2020. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/idiopathic-intracranial-hypertension
44. Bateman, G.A., Bateman, A.R. A perspective on the evidence for glymphatic obstruction in spaceflight associated neuro-ocular syndrome and fatigue. npj Microgravity 10, 23 (2024). https://doi.org/10.1038/s41526-024-00365-9
45. Roy B, Nunez A, Aysola RS, Kang DW, Vacas S, Kumar R. Impaired Glymphatic System Actions in Obstructive Sleep Apnea Adults. Front Neurosci. 2022;16:884234. Published 2022 May 6. doi:10.3389/fnins.2022.884234
46. Cerebrospinal Fluid Leak. Cedars Sinai. 2022. https://www.cedars-sinai.org/health-library/diseases-and-conditions/c/cerebrospinal-fluid-leak.html
47. Vemuri NV, Karanam LSP, Manchikanti V, Dandamudi S, Puvvada SK, Vemuri VK. Imaging review of cerebrospinal fluid leaks. Indian J Radiol Imaging. 2017 Oct-Dec;27(4):441-446. doi: 10.4103/ijri.IJRI_380_16. PMID: 29379240; PMCID: PMC5761172.
48. Pasumarthi, Anusha MSc; Luo, Annette BS; Shah, Hemali BS, BA; Carroll, Ian MD, MS. Letter: Considering Cerebrospinal Fluid Leaks in Ehlers-Danlos Patients: Raising Awareness Amongst Neurosurgeons
49. Dong C, Zhao PF, Yang JG, Liu ZH, Wang ZC. Incidence of vascular anomalies and variants associated with unilateral venous pulsatile tinnitus in 242 patients based on dual-phase contrast-enhanced computed tomography. Chin Med J (Engl). 2015;128(5):581-585. doi:10.4103/0366-6999.151648
50. Essibayi MA, Oushy SH, Lanzino G, Brinjikji W. Venous Causes of Pulsatile Tinnitus: Clinical Presentation, Clinical and Radiographic Evaluation, Pathogenesis, and Endovascular Treatments: A Literature Review. Neurosurgery. 2021 Oct 13;89(5):760-768. doi: 10.1093/neuros/nyab299. PMID: 34392338.
51. Abdalkader M, Nguyen TN, Norbash AM, Raz E, Shapiro M, Lenck S, Brinjikji W, Weber P, Sakai O. State of the Art: Venous Causes of Pulsatile Tinnitus and Diagnostic Considerations Guiding Endovascular Therapy. Radiology. 2021 Jul;300(1):2-16. doi: 10.1148/radiol.2021202584. Epub 2021 May 25. PMID: 34032509.
52. Li X, Zhao P, Qiu X, et al. Altered cerebral blood flow in patients with unilateral venous pulsatile tinnitus: an arterial spin labeling study. Br J Radiol. 2021;94(1120):20200990. doi:10.1259/bjr.20200990
53. Raz, E et al. Emergence of Venous Stenosis as a Dominant Cause of Pulsatile Tinnitus. Stroke: Vascular and Interventional Neurology. 2022. https://doi.org/10.1161/SVIN.121.000154
54. Pereira VM, Cancelliere NM, Najafi M, MacDonald D, Natarajan T, Radovanovic I, Krings T, Rutka J, Nicholson P, Steinman DA. Torrents of torment: turbulence as a mechanism of pulsatile tinnitus secondary to venous stenosis revealed by high-fidelity computational fluid dynamics. J Neurointerv Surg. 2021 Aug;13(8):732-737. doi: 10.1136/neurintsurg-2020-016636. Epub 2020 Nov 20. PMID: 33219149; PMCID: PMC8292577.
55. Hulens M, Dankaerts W, Rasschaert R, Bruyninckx F, De Mulder P, Bervoets C. The Link Between Empty Sella Syndrome, Fibromyalgia, and Chronic Fatigue Syndrome: The Role of Increased Cerebrospinal Fluid Pressure. J Pain Res. 2023;16:205-219https://doi.org/10.2147/JPR.S394321
56. Wirth, K.J., Scheibenbogen, C. & Paul, F. An attempt to explain the neurological symptoms of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J Transl Med 19, 471 (2021). https://doi.org/10.1186/s12967-021-03143-3
57. Bragée B, Michos A, Drum B, Fahlgren M, Szulkin R, Bertilson BC. Signs of Intracranial Hypertension, Hypermobility, and Craniocervical Obstructions in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front Neurol. 2020 Aug 28;11:828. doi: 10.3389/fneur.2020.00828. PMID: 32982905; PMCID: PMC7485557.
58. Higgins JNP, Pickard JD, Lever AML. Chronic fatigue syndrome and idiopathic intracranial hypertension: Different manifestations of the same disorder of intracranial pressure? Med Hypotheses. 2017 Aug;105:6-9. doi: 10.1016/j.mehy.2017.06.014. Epub 2017 Jun 24.
59. Townsend RK, Fargen KM. Intracranial Venous Hypertension and Venous Sinus Stenting in the Modern Management of Idiopathic Intracranial Hypertension. Life (Basel). 2021 May 31;11(6):508. doi: 10.3390/life11060508. PMID: 34073077; PMCID: PMC8227267.
60. Wei,D, Morrison,E. Histology, Astrocytes. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK545142/#:~:text=Astrocytes%20are%20a%20subtype%20of,barrier%2C%20and%20promoting%20synapse%20formation.
61. Larsen, K. Intracranial Hypertension: Beyond CSF. Diagnosis and Treatment. MSK Neurology. 2020. https://mskneurology.com/intracranial-hypertension-beyond-csf-diagnosis-and-treatment/
62. Fargen KM, Coffman S, Torosian T, Brinjikji W, Nye BL, Hui F. "Idiopathic" intracranial hypertension: An update from neurointerventional research for clinicians. Cephalalgia. 2023
63. Dwyer CM, Prelog K, Owler BK. The role of venous sinus outflow obstruction in pediatric idiopathic intracranial hypertension. J Neurosurg Pediatr. 2013 Feb;11(2):144-9. doi: 10.3171/2012.10.PEDS1299. Epub 2012 Nov 23. PMID: 23176141.
64. Wu L, Zhang Z, Liang X, et al. Glymphatic system dysfunction in recovered patients with mild COVID-19: A DTI-ALPS study. iScience. 2023;27(1):108647. Published 2023 Dec 7. doi:10.1016/j.isci.2023.108647
65. Zhou, Z et al. Impaired Cerebrospinal Fluid 18F-FEPPA Clearance in Long COVID Suggests Altered Glymphatic System Function. Journal of Nuclear Medicine. 2024. https://jnm.snmjournals.org/content/65/supplement_2/242407
66. Pessa JE. Identification of a novel path for cerebrospinal fluid (CSF) drainage of the human brain. PLoS One. 2023 May 4;18(5):e0285269. doi: 10.1371/journal.pone.0285269. PMID: 37141309; PMCID: PMC10159342.
67. Takasu S, Ariizumi M, Matsumoto S, Nakagawa H, Iwadate K. Cerebral venous sinus thrombosis associated with COVID-19: an autopsy case report. Forensic Sci Med Pathol. 2022 Mar;18(1):80-85. doi: 10.1007/s12024-022-00458-5. Epub 2022 Jan 24. PMID: 35067810; PMCID: PMC8784249.
68. Exelby,G The Glymphatic System MCMC-Research. 2024 https://www.mcmc-research.com/post/the-glymphatic-system
69. Mogensen FL, Delle C, Nedergaard M. The Glymphatic System (En)during Inflammation. Int J Mol Sci. 2021 Jul 13;22(14):7491. doi: 10.3390/ijms22147491. PMID: 34299111; PMCID: PMC8305763.
70. Bezerra MLS, Ferreira ACAF, de Oliveira-Souza R. Pseudotumor Cerebri and Glymphatic Dysfunction. Front Neurol. 2018 Jan 16;8:734. doi: 10.3389/fneur.2017.00734. PMID: 29387036; PMCID: PMC5775972.
71. Jones O, Cutsforth-Gregory J, Chen J, Bhatti MT, Huston J, Brinjikji W. Idiopathic Intracranial Hypertension is Associated with a Higher Burden of Visible Cerebral Perivascular Spaces: The Glymphatic Connection. AJNR Am J Neuroradiol. 2021 Dec;42(12):2160-2164. doi: 10.3174/ajnr.A7326. Epub 2021 Nov 25. PMID: 34824096; PMCID: PMC8805760.
72. Cherian I, Beltran M, Kasper EM, Bhattarai B, Munokami S, Grasso G. Exploring the Virchow-Robin spaces function: A unified theory of brain diseases. Surg Neurol Int. 2016 Oct 7;7(Suppl 26):S711-S714. doi: 10.4103/2152-7806.192486. Erratum in: Surg Neurol Int. 2016 Nov 28;7:102. doi: 10.4103/2152-7806.194815. PMID: 27857861; PMCID: PMC5093876.
73. Gouveia-Freitas K, Bastos-Leite AJ. Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology. 2021;63(10):1581-1597. doi:10.1007/s00234-021-02718-7
74. Lee H, Xie L, Yu M, et al. The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci. 2015;35(31):11034-11044. doi:10.1523/JNEUROSCI.1625-15.2015
75. Reddy OC, van der Werf YD. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain Sci. 2020;10(11):868. Published 2020 Nov 17. doi:10.3390/brainsci10110868
76. Ren X, Liu S, Lian C, Li H, Li K, Li L, Zhao G. Dysfunction of the Glymphatic System as a Potential Mechanism of Perioperative Neurocognitive Disorders. Front Aging Neurosci. 2021 Jun 7;13:659457. doi: 10.3389/fnagi.2021.659457. PMID: 34163349; PMCID: PMC8215113.
77. Shen MD. Cerebrospinal fluid and the early brain development of autism. J Neurodev Disord. 2018 Dec 13;10(1):39. doi: 10.1186/s11689-018-9256-7. PMID: 30541429; PMCID: PMC6292033.
78. Huang S, Fishell G. In SARS-CoV-2, astrocytes are in it for the long haul. Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2209130119. doi: 10.1073/pnas.2209130119. Epub 2022 Jul 18. PMID: 35858460; PMCID: PMC9335203.
79. Colinet et al. SARS-CoV2 infection triggers reactive astrocyte states and inflammatory conditions in long-term Human Cortical Organoids. BioRxiv Preprint. doi: https://doi.org/10.1101/2024.04.16.589036
80. Rosu GC, Mateescu VO, Simionescu A, Istrate-Ofiteru AM, Curcă GC, Pirici I, Mogoanta L, Mindrila I, Kumar-Singh S, Hostiuc S, Pirici D. Subtle vascular and astrocytic changes in the brain of coronavirus disease 2019 (COVID-19) patients. Eur J Neurol. 2022 Dec;29(12):3676-3692. doi: 10.1111/ene.15545. Epub 2022 Oct 1. PMID: 36056566; PMCID: PMC9539405.
81. Zhang, PP., He, ZC., Yao, XH. et al. COVID-19-associated monocytic encephalitis (CAME): histological and proteomic evidence from autopsy. Sig Transduct Target Ther 8, 24 (2023). https://doi.org/10.1038/s41392-022-01291-6
82. Wu L, Zhang Z, Liang X, et al. Glymphatic system dysfunction in recovered patients with mild COVID-19: A DTI-ALPS study. iScience. 2023;27(1):108647. Published 2023 Dec 7. doi:10.1016/j.isci.2023.108647
83. Hahn,M, New study: Risk factor for developing Alzheimer’s disease increases by 50-80% in older adults who caught COVID-19. Case Western Reserve University. 2022. https://thedaily.case.edu/new-study-risk-factor-for-developing-alzheimers-disease-increases-by-50-80-in-older-adults-who-caught-covid-19/
84. Zhou, ZA et al. Impaired Cerebrospinal Fluid 18F-FEPPA Clearance in Long COVID Suggests Altered Glymphatic System Function. Journal of Nuclear Medicine. 2024. https://jnm.snmjournals.org/content/65/supplement_2/242407/tab-article-info
85. Hepworth EJ, Love AV, Hartford RL. Intracranial Hypertension Secondary to Venous Outflow Obstruction. J Neurol Surg B Skull Base. 2021;82(Suppl 2):S65-S270. Published 2021 Feb 12. doi:10.1055/s-0041-1725347
86. Kosugi,K et al Posture-Induced Changes in the Vessels of the Head and Neck: Evaluation using conventional Supine CT and Upright CT. Nature (Scientific reports) 2020. https://www.nature.com/articles/s41598-020-73658-0
87. Teijink SBJ, Goeteyn J, Pesser N, van Nuenen BFL, Thompson RW, Teijink JAW. Surgical approaches for thoracic outlet decompression in the treatment of thoracic outlet syndrome. J Thorac Dis. 2023;15(12):7088-7099. doi:10.21037/jtd-23-546
88. de Kleijn RJCMF, Schropp L, Westerink J, van Hattum ES, Petri BJ, de Borst GJ. Functional outcome of arterial thoracic outlet syndrome treatment. Front Surg. 2023;9:1072536. Published 2023 Jan 16. doi:10.3389/fsurg.2022.1072536
89. Thoracic Outlet Syndrome. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/thoracic-outlet-syndrome/diagnosis-treatment/drc-20353994
90. Physiopedia contributors. Management of Thoracic Outlet Syndrome. Physiopedia 2024. https://www.physio-pedia.com/Management_of_Thoracic_Outlet_Syndrome
91. Zhou D, Ding JY, Ya JY, et al. Understanding jugular venous outflow disturbance. CNS Neurosci Ther. 2018;24(6):473-482. doi:10.1111/cns.12859
92. Suh DC, Kim JL, Kim EH, et al. Carotid baroreceptor reaction after stenting in 2 locations of carotid bulb lesions of different embryologic origin. AJNR Am J Neuroradiol. 2012;33(5):977-981. doi:10.3174/ajnr.A2891
94. Ketch T, Biaggioni I, Robertson R, Robertson D. Four faces of baroreflex failure: hypertensive crisis, volatile hypertension, orthostatic tachycardia, and malignant vagotonia. Circulation. 2002 May 28;105(21):2518-23. doi: 10.1161/01.cir.0000017186.52382.f4. PMID: 12034659.
95. Scholbach, T.: Diagnosis and treatment of vascular compression syndromes of the abdomen based on the anatomical features of man and gender-specific characteristics after puberty. https://scholbach.de/wp-content/uploads/2017/09/20170917-vascular-compression-syndromes-website.pdf
96. Bałabuszek K, Toborek M, Pietura R. Comprehensive overview of the venous disorder known as pelvic congestion syndrome. Ann Med. 2022;54(1):22-36. doi:10.1080/07853890.2021.2014556
97. Arun A, Amans MR, Higgins N, et al. A proposed framework for cerebral venous congestion. Neuroradiol J. 2022;35(1):94-111. doi:10.1177/19714009211029261
98. Tuță S. Cerebral Venous Outflow Implications in Idiopathic Intracranial Hypertension-From Physiopathology to Treatment. Life (Basel). 2022;12(6):854. Published 2022 Jun 8. doi:10.3390/life12060854
99. Li M, Gao X, Liu F, et al. Diastolic blood pressure predicts enlarged vertebral venous plexus and intracranial pressure in patients with bilateral transverse sinus stenosis. Front Neurol. 2022;13:957353. Published 2022 Aug 22. doi:10.3389/fneur.2022.957353
100. Tsai HH, Hsieh YC, Lin JS, Kuo ZT, Ho CY, Chen CH, Chang CF. Functional Investigation of Meningeal Lymphatic System in Experimental Intracerebral Hemorrhage. Stroke. 2022 Mar;53(3):987-998. doi: 10.1161/STROKEAHA.121.037834. Epub 2022 Feb 11. PMID: 35144488.
101. Zhang Q, Chen Y, Li Y, Feng Z, Liang L, Hao X, Kang W, Zhang Z, Zhang X, Hu R, Feng H, Chen Z. Neutrophil extracellular trap-mediated impairment of meningeal lymphatic drainage exacerbates secondary hydrocephalus after intraventricular hemorrhage. Theranostics. 2024 Feb 24;14(5):1909-1938. doi: 10.7150/thno.91653. PMID: 38505607; PMCID: PMC10945341.
102. Mangalore S, Rakshith S, Srinivasa R. Solving the Riddle of "Idiopathic" in Idiopathic Intracranial Hypertension and Normal Pressure Hydrocephalus: An Imaging Study of the Possible Mechanisms - Monro-Kellie 3.0. Asian J Neurosurg. 2019;14(2):440-452. doi:10.4103/ajns.AJNS_252_18
103. Xu JQ, Liu QQ, Huang SY, et al. The lymphatic system: a therapeutic target for central nervous system disorders. Neural Regen Res. 2023;18(6):1249-1256. doi:10.4103/1673-5374.355741
104. Jacob, L., Boisserand, L.S.B., Geraldo, L.H.M. et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun 10, 4594 (2019). https://doi.org/10.1038/s41467-019-12568-w
105. Albayram MS, Smith G, Tufan F, Tuna IS, Bostancıklıoğlu M, Zile M, Albayram O. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. 2022 Jan 11;13(1):203. doi: 10.1038/s41467-021-27887-0. PMID: 35017525; PMCID: PMC8752739.
106. Slater AM, Barclay SJ, Granfar RMS, Pratt RL. Fascia as a regulatory system in health and disease. Front Neurol. 2024;15:1458385. Published 2024 Aug 7. doi:10.3389/fneur.2024.1458385
107. Tuckey B, Srbely J, Rigney G, Vythilingam M, Shah J. Impaired Lymphatic Drainage and Interstitial Inflammatory Stasis in Chronic Musculoskeletal and Idiopathic Pain Syndromes: Exploring a Novel Mechanism. Front Pain Res (Lausanne). 2021 Aug 23;2:691740. doi: 10.3389/fpain.2021.691740. PMID: 35295453; PMCID: PMC8915610.
108. Deng,J et al. Head and neck lymphoedema and fibrosis: a case study. Journal of Lymphoedema 2018. https://woundsinternational.com/wp-content/uploads/2023/02/jol_13-1_24-28_deng-web.pdf
Comments