The Glymphatic System
- Graham Exelby
- Dec 30, 2023
- 33 min read
Updated: Dec 1, 2024
Glymphatic System (or glymphatic clearance pathway or paravascular system)
Dr Graham Exelby. January 2024- Currently under revision
Introduction
The glymphatic system, first described in 2013, is a macroscopic system for waste clearance in the brain, or in simple terms, the brain’s sewer. It uses a system of perivascular channels, formed by astroglial cells, to promote efficient elimination of soluble proteins and metabolites from the CNS. The name is in reference of its dependence on glial cells and the similarities to the functions of the peripheral lymphatic system. Initially thought to provide the solution to how sensitive neural tissue of the CNS functions, it has since been established there are also conventional lymphatic vessels lining dural sinuses and meningeal arteries. The glymphatic systems in the brain and eye export fluid and solutes from metabolically active neural tissue. Fluids from both the brain and the eye drain via the cervical lymph vessels, which empty into the venous system at the level of the subclavian veins.(6)
Figure 1. The Glymphatic System
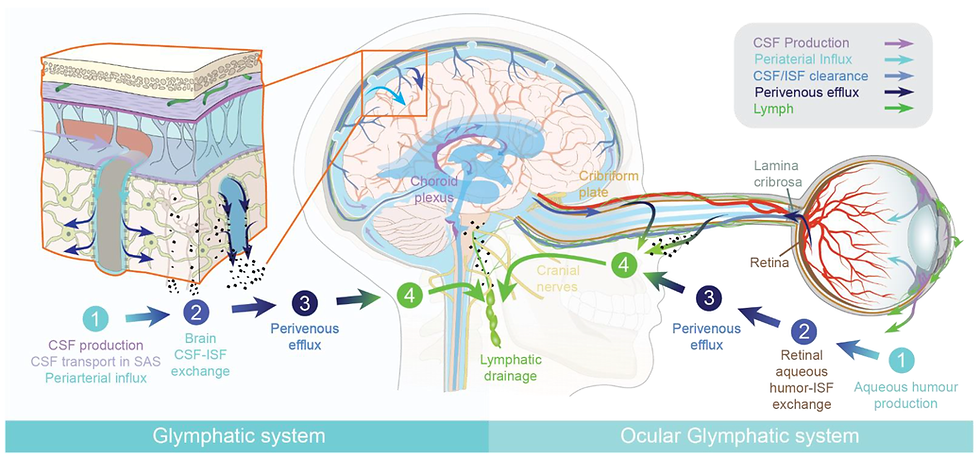
Source: Mogensen et al. The Glymphatic System (en)during Inflammation (6)
Besides eliminating waste, the glymphatic system may also distribute non-waste compounds, such as glucose, lipids, amino acids, and neurotransmitters, as well as permitting the flow of fluid through the brain. The glymphatic system functions mainly during sleep and is largely disengaged during wakefulness. The biological need for sleep across all species may therefore reflect that the brain must enter a state of inactivity that enables elimination of potentially neurotoxic waste products, including β-amyloid.” (2)
The ongoing research into glymphatics could shed light on POTS and long COVID. Professors Marshall-Gradisnik and Smith’s team at Griffith University in Queensland discovered mutations in an important TRP pathway that affects the function of the Natural Killer Cells and glymphatics. Low Dose Naltrexone improves function, a valuable tool in management of the cognitive changes and chronic fatigue by improving glymphatic flow. The exact mechanism for this is uncertain, but does appear to reduce TLR2 expression in the brain and act on the astrocyte dysfunction and glial activation with direct interactions with TLR4.(65)
Glymphatic Dysfunction:
Glymphatic system is the brain’s sewer, clearing toxins, and distributing non-waste products
Glymphatic flow is very important when fatigue and cognitive impairment are present
Function affected by impaired intracerebral arterial flow and sleep disorder
Function affected by Covid via TRP pathway (probably reflects underlying TLR4 mutation and subsequent dysfunctional immune response in ME/CFS and POTS)
Function is affected by impaired astrocyte/glutamate dysfunction- astrocytes for the paravascular channels that are important in maintaining drainage
Physical “drivers” can impact on function especially neck posture –phones and computer usage in particular
LDN (low dose naltrexone) can improve Natural Killer Cell and glymphatic function. (66) This appears to modulate both TLR2 and TLR4, mechanism not known but subject of intense study at Griffith University
Lymphatic obstruction appears to be critical in increasing CSF pressure. This usually improves with good posture, management of Thoracic Outlet Syndrome and Jugular Outlet Syndrome if present·
Glymphatic Functioning
“Cerebrospinal fluid flows into the paravascular space, (also known as perivascular spaces or Virchow-Robin spaces,) around cerebral arteries, combining with interstitial fluid and parenchymal solutes, exiting down venous paravascular spaces. Exchange of solutes is driven primarily by arterial pulsation and regulated during sleep by the expansion and contraction of brain extracellular space. (1)(2)
Besides eliminating waste, the glymphatic system may also distribute non-waste compounds, such as glucose, lipids, amino acids, and neurotransmitters, as well as permitting the flow of fluid through the brain. Intriguingly, the glymphatic system functions mainly during sleep and is largely disengaged during wakefulness.
Xie et al in 2013 (2) described that the biological need for sleep across all species may therefore reflect that the brain must enter a state of inactivity that enables elimination of potentially neurotoxic waste products, including β-amyloid.” (2)
The glymphatics also play an important role in the paravascular transport of lipids and impairment of glymphatic circulation results in intracellular lipid accumulation and pathological signalling among astrocytes. Glymphatic dysfunction has been shown in animal models of traumatic brain injury, Alzheimer's disease, and stroke. (5) It is also potentially involved in haemorrhagic and ischaemic neurovascular disorders and other acute degenerative processes such as normal pressure hydrocephalus and traumatic brain injury.(7)
Microglial activation including Covid and the link to Glutamate Dysfunction
Coronavirus disease 2019 (COVID-19) is the greatest public health crisis in the early 21st century. Its causative agent, Severe Acute Respiratory Syndrome (ARDS) coronavirus 2 (SARS-CoV-2), is an enveloped single stranded positive-sense ribonucleic acid virus that enters cells via the angiotensin converting enzyme 2 receptor or several other receptors. While COVID-19 primarily affects the respiratory system, other organs including the brain can be involved.
SARS-Co-2 activates threat receptors, or Toll-Like Receptors (in particular TLR2 and TLR4 where mutations have been found,) triggering a dysfunctional immune response provoking the excessive cytokine storm with interleukin 6 (IL-6) and tissue necrosis factor alpha (TNFα) that sensitise microglial cells with consequent small-fibre neuropathy which in turn causes autonomic instability and other neuropathic symptoms.(20)(21)
Inflammatory microglial activation (IL-6 and TNFa) is the most common brain pathology found in patients who died of COVID-19: 42% are affected, and another 15% have microclots in brain tissue.(23) Dong et al (24) demonstrated that brain inflammation plays a critical role in the pathophysiology of brain diseases. Microglia, the resident immune cells in the brain, play an important role in brain inflammation, while brain mast cells, rather than microglia, are the "first responders" to brain injury. They showed that site-directed injection of a “mast-cell degranulator” compound in the hypothalamus initiated the acute inflammatory response by inducing mast-cell degranulation, activating microglia, and triggering the production of inflammatory factors.
Microglia account for 10% to 15% of all cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defence in the CNS.(25) The consequence of the microglial sensitisation that causes small fibre neuropathy is abnormal functioning of the autonomic nervous system, that controls everyday body activities- autonomic dysfunction or dysautonomia. Symptoms of this include: lightheadedness, presyncope, abnormal heart rhythms, shortness of breath, dry eyes, loss of saliva, temperature dysregulation, excessive sweating, constipation or diarrhoea, nausea, unexplained anxiety, abnormal bowel and bladder function, and other symptoms.(26) Many have a “cross-over” with mast cell activation (27)(28) and mechanical effects on the vagus, brainstem, sympathetic ganglia and other areas.
The complex nature of the immune response and mast cell activation in now an integral part of Long Covid pathogenesis. The same microglial activation has been demonstrated in other conditions -CFS (31), ADHD (30), migraine, Fibromyalgia syndrome (40) and Endometriosis (29).
Glutamate Dysfunction, astrocyte dysfunction and PEMT mutations- a critical component?
Blood flow in the brain is regulated by neurons and astrocytes, “a subtype of glial cells that make up the majority of cells in the human central nervous system. Astrocytes perform metabolic, structural, homeostatic, and neuroprotective tasks such as clearing excess neurotransmitters, stabilizing and regulating the blood-brain barrier, and promoting synapse formation. Because astrocytes fulfill many essential functions, their dysfunction has implicated them in several neurological disorders.”(32)
SARS-CoV-1 infects and propagates in astrocytes, while neurons and microglia are less likely to be directly infected. From post-mortem studies, SARS-CoV-2 activates a huge reactive response in glial cells, and not via direct infection.(33) An emergent hypothesis of glutamate/astrocyte dysfunction by Guedl (34)(35) in Long Covid provides a potential explanation for impaired glymphatic function and potential effects on symptoms such as fatigue, brain fog and head pressure.
Microglia and astrocytes play essential roles in the central nervous system contributing to many functions including homeostasis, immune response, blood–brain barrier maintenance and synaptic environment. There is cross-talk between them and astrocytes influence and coordinate each other and their effects on the brain environment. Microglia robustly express a wide range of TLRs whereas astrocytes preferentially express TLR3 receptors. Studies suggest that the astrocyte/microglial cholesterol metabolism in the brain could be a key feature of neurodegenerative disorders, and has been implicated in fatigue and cognitive impairment (36) as with PEMT mutations.(38)
Attwell et al (37) describe “It is now recognized that neurotransmitter-mediated signalling has a key role in regulating cerebral blood flow, that much of this control is mediated by astrocytes, that oxygen modulates blood flow regulation, and that blood flow may be controlled by capillaries as well as by arterioles.” The astrocyte/microglial cross-linking is well demonstrated by Palpagama, Waldvogel and Kwakowsky (64) in their work in neuroinflammation in the Huntington’s Disease (HD). This is characterized by a reactive changes in these glial cells and they describe activated microglia and reactive astrocytes contributing to HD pathology through transcriptional activation of pro-inflammatory genes to perpetuate a chronic inflammatory state. These reactive astrocytes also display functional changes in glutamate and ion homeostasis and energy metabolism. The astrocytic and microglial changes are thought may contribute to the neuronal death observed with the progression of HD.
Glutamate is a key excitory neurotransmitter with critical roles in multiple brain functions and synaptic plasticity. Glutamine synthetase is an enzyme in astrocytes that breaks down glutamate into glutamine.(36) In excess it has been linked to many neurodegenerative diseases eg Alzheimer’s Disease. Glutamate toxicity has been associated with severe stress, and in the development of many psychiatric disorders including schizophrenia and bipolar disease.(39) Fibromyalgia with its impaired pain perception pathways, has notably been found to have higher concentrations of glutamate, in regions of the brain implicated in processing pain information.(40) Visual snow is another that has been linked to abnormal glutamate and serotonin functioning. (41) Autism is considered to have the same microglial activation as POTS, fibromyalgia, chronic fatigue, ADHD (30) and endometriosis (40), but it has been localized to the astroglial/glutamate dysfunction (42)(43)(44) as proposed by Guedj and associates in Long Covid,(34)(35) so the presence of comorbidities known to be associated with this dysfunction implicates possible astrocyte/glutamate dysfunction in these patients.
The glymphatic system, which uses a system of perivascular channels formed by astroglial cells . The Guedj hypothesis of astrocyte and glutamate dysfunction, matched with the known work in autism and visual snow makes a strong case for astroglial/glutamate associated glymphatic dysfunction to be part of the central core of POTS. The considerable increase in ADHD as a POTS co-morbidity may lie in the combination of astrocyte damage and microglial activation.
Phosphatidylethanolamine N-methyltransferase (PEMT) is involved in the biosynthesis of phosphatidylcholine (PC) from phosphatidylethanolamine (PE), and likely to be the underlying culprit in persistent D-Dimer tests in Long Covid. PEMT mutations are associated with fatigue, fatty liver, and increased neurodegenerative risk.
Fatigue is a common symptom of glymphatic dysfunction. PEMT mutations and its associated mitochondrial dysfunction, and is thought to be involved in neurodegenerative disease.(38) The linking of PEMT with glutamate dysfunction creates a valuable area for future research. At present, discussions with a pathologist confirmed there are no biomarkers for the clinician to assess this mutation.
Clinical Findings associated with POTS
To summarize the clinic findings in POTS, there are a series of vascular compression syndromes that we have found in all POTS (over 350). Head and neck vascular and mechanical pathology underpins 85% of POTS and drives symptoms, with impaired venous, arterial and lymphatic flow in the head and neck, and consequent vascular flow changes in the brainstem and brain proper. These appear responsible for Intracranial Hypertension, Intracranial Hypotension with CSF leaks, and Intracranial Vascular Pressure Dysfunction.
Jugular outlet syndrome (JOS) , (Venous Eagle, or Styloidogenic Jugular Venous Compression Syndrome) is very common finding in our POTS vascular compression studies with very high level of correlation where the Internal Jugular Vein (s) is compressed between the transverse process of the first cervical vertebra and the stylohyoid ligament, with varying levels of compression. The preliminary studies suggest that Venous Thoracic Outlet Syndrome accompanies these, with a strong correlation between VTOS, JOS and cervical spine pathology, potentially increasing intracranial vascular pressure. Arterial Thoracic Outlet Syndrome is thought also to increase the level of intracranial vascular pressure.
While case studies have found changes in head and neck vascular and musculoskeletal function in most POTS, there are many with intra-abdominal compression areas. Increasingly there is a high incidence of Median Arcuate Ligament Syndrome (MALS), Superior Mesenteric Artery Syndrome (SMA) as well as the Nutcracker and May-Thurner Syndromes presenting as POTS. This concurs with the increased incidence noted between pre-Covid and post-Covid patient numbers.
We cannot as yet ascertain if these “rare diseases, especially MALS and SMA” signify the microglial -induced small fibre neuropathic central sensitization, or a change in collagen or both. Improved radiological methods may also be a significant factor. As we have no way to confirm these, so this remains a series of comparative observations.
The “mechanical and hydraulic” causes we have found in clinic are described as:
·Thoracic Outlet Syndrome (TOS). Arterial TOS can have direct effects on cerebral circulation. Venous TOS is clinically and functionally directly related to JOS and cervical spine dysfunction, generally from poor posture and trauma – Thoracic Outlet Syndrome
Jugular Outlet Syndrome (JOS) where the Internal Jugular Vein (s) is compressed between the transverse process of the first cervical vertebra and the stylohyoid ligament. Jugular Outlet Syndrome is intricately linked to the Thoracic Outlet Syndrome and upper cervical pathology. Internal Jugular Vein Stenosis (IJVS) and Internal Jugular Vein Obstruction (IJVO)- (46)(45)(47) collectively with Jugular Outlet Syndrome, both affect venous outflow from the brain,(48) but the jugular dilation of the Internal Jugular Vein potentially affects the vagus, carotid baroreceptors, cervical sympathetic chain and jugular nerve.
Collectively the JOS and IJVS has been referred to as chronic cerebrospinal venous insufficiency (CCSVI) (45). Dynamic scanning of the Subclavian and Internal Jugular veins in a small preliminary study of 15 has shown the Internal Jugular Vein to dilate as the arms are elevated, and when neck flexion is added, obstruction to Internal Jugular Vein flow has been shown, the IJV flow return slow to return. These results mirror the findings by van Campen, Rowe and Visser (63) in middle cerebral artery flow in CFS. As this has been accompanied by POTS symptoms, this requires formal studies to confirm the importance of this finding, and to differentiate the relative importance of each facet -Internal Jugular Vein Dysfunction- Jugular Outlet Syndrome, Internal Jugular Vein Stenosis and Obstruction.
Loss of cervical lordosis/ flexion kyphosis – potentially impacting on Vertebral Artery flow as found by Bulut (50), Vertebral Vein and surrounding lymphatics- Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular and Lymphatic Dysfunction.
All the above are likely to cause lymphatic obstruction as these in particular surround the Internal Jugular and Vertebral Veins. This impaired lymphatic flow potentially creates “backpressure” in the Glymphatic System which is affected by genetic predisposition, sleep disorder, and most importantly, Covid infections. Glymphatic System
The combination of these changes is described in Intracranial Hypertension, Intracranial Hypotension, CSF Leaks and Craniovascular Pressure Change
In the abdomen the primary ones involve the Coeliac axis (MALS and SMA), Renal Vein Compression with gonadal vein reflux (Nutcracker Syndrome) with pelvic congestion, and May-Thurner Syndrome involving the iliac veins. Recent advances in radiology has shown a high incidence of left renal vein compression associated with Superior Mesenteric Artery Syndrome (SMA), providing a potential explanation for intra-abdominal venous (and spinal vein plexus) dysfunction in SMA, previously unable to be explained. Bowdino, Owens and Shaw (51) describe that in 3% of people, the retroperitoneal venous vessels, such as lumbar or hemiazygos vessels, drain into the right renal vein before it enters the inferior vena cava. The venous congestion potentially involves the Azygous and spinal vein systems- Intra-abdominal Vascular Compression Syndromes
The Azygous system of veins, which includes the hemiazygous and accessory hemiazygous veins provide an alternative blood flow from the lower half of the body to the superior vena cava was recognized by Nicolaides et al as significant in their work on venous outflow abnormalities and MS (45) and explored by Scholbach.(62) This, and its association with the vertebral venous system has a place in POTS pathogenesis, but as yet this has not been fully elucidated. Symptoms occasionally can only be explained by dysfunctional azygous flow, but again we have no evidence to confirm this. Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular Dysfunction, Intra-Abdominal Vascular Compression Syndromes
Lymphatic obstruction affects glymphatic function and cerebral dysfunction
Natale et al (4) describe “the glymphatic pathway is connected to a classic lymphatic network, associated with dural meninges covering the brain, as well as sheaths of cranial nerves, or drains via the olfactory route, then exiting through cranial foramina. This drains ultimately to deep and superficial lymph nodes.” They explain that “during ageing meningeal lymphatic vessels exhibit decreased vessel diameter and reduced drainage to cervical lymph nodes. Experimental studies in mice showed that ablated or ligated meningeal lymphatics led to an increase in b-amyloid deposition and macrophage recruitment to plaque sites, with a reduced extracellular clearance of altered proteins.” (4)
Natale et al (4) describe how “not only the level of consciousness, but also body posture contributes to drainage.” Lee et al (16) showed in rat studies that glymphatic transport was most efficient in the lateral position compared with the supine or prone positions.
Lymphatics of the face and head drain inferiorly into the pericervical lymphatic collar. This collar consists of a series of connected lymph nodes, which form a chain that encircles the junction of the head and the neck. The collar consists of the following groups of nodes (from posterior to anterior): occipital, postauricular (retroauricular), preauricular, submandibular, and submental. These lymph nodes are drained by lymphatic channels that eventually drain into the deep cervical lymph nodes, located along the internal jugular vein. The deep cervical lymph nodes empty into the thoracic duct on the left side and the right lymphatic duct on the right side.
There are no valves between the lymphatics and the glymphatic system. Obstruction of the lymphatics in the neck will cause a “backup” of pressure and potentially causing Idiopathic Intracranial Hypertension.
Figure 2. Lymph Chains Head and Neck

Source: drzero. Cervical Adenopathy and Neck Masses. 2019 Radiology Key. https://radiologykey.com/cervical-adenopathy-and-neck-masses/
Glymphatic drainage and association with Dysautonomia (and POTS)
Boisserand et al (8) confirmed in mice studies that “vertebral lymph vessels connect to peripheral sensory and sympathetic ganglia and form similar vertebral circuits connecting to lymph nodes and the thoracic duct. They showed that the connection between lymph vessels and sympathetic ganglia occurred at the surface of the ganglia revealing a hitherto unknown anatomical interaction between the autonomous nervous system and vertebral lymphatic vessels. They are closely apposed around the chains of sensory and sympathetic nervous ganglia, so lymphatic vessels may provide molecular signals to the sympathetic neurons that control vascular tone of lymphatic ducts and cerebral arteries and arterioles.”
“Previous observations by the authors also showed that adrenergic fibres connect to the thoracic lymphatic duct and also innervate the wall of lymph node arterioles. The crosstalk between spine LVs and the sympathetic system is thus likely relevant for the regulation of peripheral lymph and glymphatic drainage and may coordinate them with the activity of brain and spine tissues. The authors speculate that a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage.”(8)
Albayram et al (9) showed “dural lymphatic structures along the dural venous sinuses in dorsal regions and along cranial nerves in the ventral regions in the human brain and they detected direct connections between lymphatic fluid channels along the cranial nerves and vascular structures and the cervical lymph nodes. They also identified age-related cervical lymph node atrophy and thickening of lymphatics channels in both dorsal and ventral regions, findings which reflect the reduced lymphatic output of the aged brain.”(9)
Fig. 3. Schematic model of the brain lymphatic system and its drainage pathways. The green signal at the orifices of neural foramina represents lymphatic fluid

Source: Albayram, M.S., Smith, G., Tufan, F. et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes.
“Macromolecules, waste products, and excess fluid from most tissues are known to drain into the systemic lymphatic system. Classically, absorption of CSF occurred through arachnoid granulations and villi of the intracranial and spinal venous sinuses. More recent animal studies have demonstrated CSF-ISF drainage via meningeal lymphatic vessels and along the cranial nerves into deep cervical lymph nodes.” According to their study result, the vascular-carotid space in the neck is very important for the CSF-ISF drainage from the brain.”(9)
Figure 4. Model of cranial nerve, dura and dural lymphatic flow at cranial foramina

Source: Albayram, M.S., Smith, G., Tufan, F. et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes
The authors suggested “that soluble waste may move from the brain parenchyma via perivascular and paravascular routes to the closest subarachnoid space and then travel along the dura mater and/or cranial nerves. They also believed that meningeal lymphatics and perineural drainage pathways are not separate systems, but rather are part of the same waste management pipeline; however, direct CSF-ISF drainage in the cranial nerve itself via the endoneurium, running adjacent to nerve axons.”(9)
Figure 5. CSF Circulation and Perivascular Space
Mark Shen (13) describes “Schematic of CSF circulation, CSF outflow systems, and the anatomy of various CSF compartments. CSF is produced by the choroid plexus in the ventricles, where it delivers growth factors to progenitor cells that originate on the surface of the ventricles, and then proliferate into neurons and migrate to form the cerebral cortex. CSF circulates from the lateral, third and fourth ventricles to the cisterns of the brain, and then flows into the subarachnoid space, where it envelops the cortical convexities of the brain (EA-CSF).”
Inset box: “From the subarachnoid space, there is retrograde influx of CSF into the parenchyma, where CSF and interstitial fluid interact in the perivascular space, alongside blood vessels that course throughout the brain. Astrocytes lining the perivascular space aid in transporting fluid that removes inflammatory waste proteins (e.g., Aβ), which are continually secreted by neurons as by-products of neuronal activity and would otherwise build up in the brain. Finally, fluid carrying these inflammatory waste products returns to the subarachnoid space (EA-CSF) and drains into meningeal lymphatic vessels and arachnoid granulations.”(13)

Source: Mark D. Shen - Shen MD. Cerebrospinal fluid and the early brain development of autism. J Neurodev Disord. 2018;10(1):39. Published 2018 Dec 13. https://dx.doi.org/10.1186%2Fs11689-018-9256-7, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=79736902 (13)
Natale et al (56) describe “the glymphatic pathway is connected to a classic lymphatic network, associated with dural meninges covering the brain, as well as sheaths of cranial nerves, or drains via the olfactory route, then exiting through cranial foramina. This drains ultimately to deep and superficial lymph nodes.” They explain that “during ageing meningeal lymphatic vessels exhibit decreased vessel diameter and reduced drainage to cervical lymph nodes. Experimental studies in mice showed that ablated or ligated meningeal lymphatics led to an increase in b-amyloid deposition and macrophage recruitment to plaque sites, with a reduced extracellular clearance of altered proteins.”(56)
Natale et al (56) continue that disruption of the glymphatic system plays a crucial role in age-related brain dysfunction, and there is strong evidence documenting the clearance of b-amyloid and tau via this system, as well as potentially harmful metabolites. In obstructive sleep apnoea they describe increasing cerebral aggregation and increased neurodegeneration. They also describe how an altered glymphatic function may account for idiopathic normal pressure hydrocephalus. “These pathological conditions are associated with a decrease in CSF influx to the glymphatic pathway or reduced clearance efficacy.” (56) Mouse studies have confirmed clearance from the meningeal lymphatics into the cervical lymphatic chains. (59)
Natale et al (56) describe how “not only the level of consciousness, but also body posture contributes to drainage.” Lymphatics of the face and head drain inferiorly into the pericervical lymphatic collar. This collar consists of a series of connected lymph nodes, which form a chain that encircles the junction of the head and the neck. The collar consists of the following groups of nodes (from posterior to anterior): occipital, postauricular (retroauricular), preauricular, submandibular, and submental.
These lymph nodes are drained by lymphatic channels that eventually drain into the deep cervical lymph nodes, located along the internal jugular vein. The deep cervical lymph nodes empty into the thoracic duct on the left side and the right lymphatic duct on the right side. It is an easy leap of faith to see that when the jugular and/or subclavian vein is compressed, then these lymphatics are also affected. There are no radiological studies to confirm this, but these can be seen clinically.
Natale et al (56) further describe the bi-directional connection between the CNS and peripheral immune system through meningeal and cervical lymphatics. “Peripherally activated T-cells can enter the brain by crossing all CNS barriers including the blood-CSF, blood-leptomeningeal and blood-brain barrier. In keeping with this, resection of either meningeal lymphatics or deep cervical lymph nodes is beneficial in models of multiple sclerosis.” The autoimmune mechanisms look to be implicated in neurodegenerative diseases such as Parkinson’s Disease. These processes are occurring with the microglial activation and cytokine release, and drainage to peripheral lymph nodes can trigger an autoimmune response.
Perineural spaces surround the cranial nerves, including the vagus to provide some level of CSF drainage to peripheral lymphatics. Natale et al (56) describe how “some insights can be provided by the ocular glymphatic system.” “Retrograde CSF inflow to the paravascular spaces in the optic nerve and eye to CSF pathway supports clearance of waste products from the retina and vitreous. This occurs in the opposite direction compared to CSF drainage, and neural activity seems to play a role on the rate of fluid fluxes, as light stimulation promotes fluid drainage and b-amyloid clearance….via the paravenous space and subsequently drained to lymphatic vessels.(56)
The research by Boisserand et al (8) and Albayram et al (9) open the door to a possible explanation for the brainstem hypoperfusion in POTS, CFS and Long Covid, via triggering of the sympathetic nervous system by compression of lymphatics in the neck affecting a regulatory loop may link meningeal lymph vessels, sympathetic chain neurons and both CNS and peripheral fluid drainage as suggested by Boisserand et al.(8) Complementing this is research from Bulut et al (50) demonstrating impaired vertebral artery function when there is a loss of cervical lordosis.
Hypothesis on underlying causes of the head pressure and brain fog in POTS- Intracranial Pressure Dysfunction- linking the systems
Intracranial hypertension is increased pressure within the skull, when there is an imbalance between the production and absorption of cerebrospinal fluid (CSF), the fluid that surrounds and cushions the brain and spinal cord. Normally, the brain and CSF are in a state of equilibrium, with the pressure inside the skull maintained within a certain range. However, various conditions can disrupt this balance.
Swetlana Blitshteyn (60) describes: “abnormal cerebral blood flow has been at the core of POTS pathophysiology, with findings of reduced cerebral perfusion, impaired cerebral autoregulation, oscillatory cerebral blood flow, which is linked to impaired cognitive function, and altered EEG amplitude modulation that may reflect abnormal brainstem physiology……As a result of cerebral hypoperfusion, cerebral tissue oxygenation … is found to decrease during orthostatic provocation in patients with POTS.” Similar abnormal neuroimaging has been demonstrated in CFS, a syndrome comorbid with POTS and sharing a significant clinical overlap. (60)
Geddes (61) demonstrated the carotid baroreceptors to be the main drivers of adrenergic POTS. Baroreceptors and mechanoreceptors respond to changes in pressure or stretch in blood vessels within the aortic arch and carotid sinus. The baroreceptors of the aortic arch transmit signals via the vagus nerve to the solitary nucleus of the medulla. The baroreceptors of the carotid sinus, where the common carotids bifurcate, transmit signals via the glossopharyngeal nerve to the solitary nucleus of the medulla.(61) Dilatation of the internal jugular vein in the carotid sheath has the potential to be a major “driver” through direct pressure on the carotid baroreceptors and at the jugular outlet, the glossopharyngeus.
“Brain fog” is a characteristic symptom in POTS, fibromyalgia and Long Covid. It can often be controlled through conservative measures: dietary and lifestyle alterations, non-prescription medication to stabilise mast cells, and improved posture. The effects of these simple interventions demonstrate the importance of the DNA, inflammatory and mechanical factors in both POTS and long COVID. We believe the glymphatic dysfunction underpins the “brain fog” especially when fatigue is also prominent.
Head “pressure” is commonly associated with this, and can be very severe and disabling, and commonly co-exists with other headache types, especially migraine and occipital neuropathy. Our findings suggest this largely represents increased intracranial pressure from impaired glymphatic system function, both within the glymphatic system itself, but also from structural obstruction to lymphatic flow in the head and neck, as well as increased arterial pressure in an Arterial Thoracic Outlet Syndrome (ATOS), and back pressure from the combination of Venous Thoracic Outlet Syndrome, venous Jugular Outlet Syndrome, Internal Jugular Vein Stenosis/Obstruction and cervical spine dysfunction.
Researchers such as Hulens (18) and Bragée (17) are looking at Intracranial Hypertension as a common pathology in POTS, CFS and Fibromyalgia. When there is no evidence of this it has been assumed there are CSF leaks. While this does appear to be occurring, and can be seen in occasional patients, especially in patients with Ehlers-Danlos Syndrome and after upper cervical spine trauma, and particularly after symptom improvement with “blood patches,” it does seem more likely that this pattern of symptoms may represent flow abnormalities in the vascular and glymphatic systems.
We have demonstrated venous obstruction can occur in Venous Thoracic Outlet Syndrome (VTOS), and most importantly, in the Vertebral veins in what is largely a postural problem, especially if there is co-existent spinal venous flow dysfunction as championed by Prof Scholbach.(62) Clinically this very important triad, the TOS, JOS and impaired vertebral flow appears largely a product of sustained poor posture and mechanical function, and the evolving management programs concur with this. There are many variations of this but all reflect multiple areas of mechanical/hydraulic dysfunction.
Within the patient cohort, the typical “pressure with brain fog “ tends to be present on standing, rather than the classical increase in ICH when recumbent. This simple finding, and especially if combined with pulsatile tinnitus generally points investigations towards CSF leaks and craniovascular pressure dysfunction from venous and lymphatic compression. This is described in more detail in:
Cervical Spine Abnormality, Ehlers-Danlos Syndrome and Vertebral Vascular and Lymphatic Dysfunction.
Intracranial Hypertension, CFS Leaks, Intracranial Hypotension and Craniovascular Pressure Change
The lymphatics are closely related to the Internal Jugular Veins and the Vertebral Veins, so when one is impacted, so too is the other. Again this is by largely by clinical assessment. As glymphatic/lymphatic obstruction increases, this can often be seen with subtle MRI brain and high level retinal photography. Overt changes are usually easily recognised, eg papilloedema, but less subtle changes eg venous distention or arterial tortuosity may be seen.
The findings of Boisserand et al (8) and Albayram et al (9) establish a direct relationship between dysfunctional venous sinus flow and the glymphatic system dysfunction, as seen in increased para-vascular spaces. The potential for autonomic symptoms caused by the sympathetic ganglia that surround the lymph channels is present, but at present unable to be quantified.
Symptoms of sub-clinical intracranial pressure change can be caused by the vascular and lymphatic flow abnormalities in the head and neck, potentially compounded by spinal vein flow change (62) in Nutcracker, May-Thurner Syndromes. The lymphatic/ sympathetic connection to the cervical sympathetic chain and with compression at the craniocervical junction is likely to cause vasoconstriction in brainstem complicating posture-caused reduced vertebral A flow.
For most patients, it requires a combination of genetic predisposition, “activation” of POTS by one of many potential causes, increasingly from SARS-CoV-2, sensitisation and then the impact of the musculoskeletal, especially craniocervical instability, and vascular dysfunction, diet, stress and other “drivers”.
The Glymphatic system’s waste clearance function is affected adversely by Covid and this may potentiate dysfunction when mechanical obstruction is already present, potentially from astrocyte/glutamate dysfunction as well as cytokine-induced microglial activation and small fibre neuropathy. Addressing these mechanical causes can be a major factor in recovering the Long Covid patients who have symptoms reflecting intracranial hypertension and Covid-induced dysautonomia. The DNA mutations better recognized in neurodegenerative disease , eg PEMT and APO E4 when present would impact on this function further. PEMT may be considered if there is accompanying fatty liver disease.
Brainstem, Dysregulation of Noradrenaline/ Locus Coeruleus Axis and Impacts on Glymphatic function
Ioachim et al (67) found significant differences between fibromyalgia patients and control patients in the connectivity of the brainstem/spinal cord network, involving the regions of the hypothalamus, thalamus, hypothalamus, locus coeruleus, and other areas. This network and the nucleus solitarius provide ample scope for ongoing research into the exact mechanism that occurs in the brainstem, and the manner in which physical problems sensitize the brainstem. Clinically, as the sensitization is reduced and the mechanical problems better managed, symptoms subside.
The locus coeruleus (from the Latin for “blue spot,”) communicates closely with the amygdala. The locus coeruleus is a cluster of noradrenergic neurons in the upper dorsolateral pontine tegmentum and is the brain’s main source of the neurotransmitter norepinephrine. This chemical is released in response to pain or stress, stimulating what is referred to as the “fight-or-flight” mechanism. In the brain, norepinephrine is a neurotransmitter; but in the rest of the body, it acts as a hormone and is released by the adrenal glands.(68)
Figure 6. Locus coeruleus

Source : Diego69 - http://www.baillement.com/anatomie/systemes.html, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=9446628
The LC-NE (norepipinephrine) system has a major role in arousal, attention, and stress response. In the brain, NE may also contribute to long-term synaptic plasticity, pain modulation, motor control, energy homeostasis, glymphatic regulation and control of local blood blow. The LC is severely affected in neurodegenerative disorders such as Alzheimer disease and Parkinson disease.
Dysregulation of LC-NE system has been implicated in sleep and arousal disorders, attention deficit hyperactivity disorder, and post-traumatic stress disorder. Extrasynaptic norepinephrine (noradrenalin) mediates signalling effects on neurons, glial cells, and microvessels.(68) It is also implicated in the dysregulation of “glymphatic” function.
Sullen et al (69) describe traumatic brain injury is an increasingly important problem in athletes and returned service personnel, highlighted by the public awareness of the dangers of Rugby and other sports. Sleep disorders and increased accumulation of beta amyloid (Aβ) and phosphorylated tau (ptau) in the paravascular spaces and along interstitial pathways in chronic traumatic encephalopathy, related to impaired glymphatic function. Glymphatic clearance relies on CSF, interstitial fluid and astrocytic processes, potentiated during sleep, and the impaired function and appears to link the various factors affecting glymphatic function including COVID-19, sleep disorder, glutamate dysfunction, lymphatic obstruction, Locus Coeruleus dysfunction and craniovascular dysfunction to the pressure and brain fog in POTS and its comorbidities.
Natale et al (4) continue “that disruption of the glymphatic system plays a crucial role in age-related brain dysfunction, and there is strong evidence documenting the clearance of b-amyloid and tau via this system, as well as potentially harmful metabolites. In obstructive sleep apnoea they describe increasing cerebral aggregation and increased neurodegeneration.
In haemorrhagic stroke, fibrin and other blood products occlude perivascular spaces, while “in ischaemic stroke there is an impaired CSF inflow and the release of several pro-inflammatory cytokines.” They also describe how an altered glymphatic function may account for idiopathic normal pressure hydrocephalus. “These pathological conditions are associated with a decrease in CSF influx to the glymphatic pathway or reduced clearance efficacy.”(4)
Figure 7. Neuroinflammation impairs glymphatic function and exacerbates the inflammatory response.

Source: Mogensen et al. The Glymphatic System (en)during Inflammation (6)
The Carotid Space
The carotid artery, jugular vein, vagus and jugular nerves and cranial nerves are located in this space. The cervical sympathetic ganglion lies in close proximity. These are then vulnerable to the compressive and flow disruptions of the Internal Jugular Vein Stenosis and Jugular Outlet Syndrome, and the vertebrals to impaired cervical shape and hypermobility. I believe the studies by Boisserand et al (8) and Albayram et al (9) provide evidence of the importance of lymphatic flow and how impairment may result in autonomic instability, as well as have a role in intracranial hypertension, and may be a major component of the autonomic chaos that is POTS.
Figure 8. The Carotid Space- defined by the carotid sheath, is a connective tissue boundary in the neck. Illustration demonstrating the contents and configuration of the left carotid space, including cranial nerves glossopharyngeus (IX), vagus (X), accessory (XI), and (XII hypoglossal) with proximity of the Cervical Sympathetic Chain

Source: Chengazi, H.U., Bhatt, A.A. Pathology of the carotid space. Insights Imaging 10, 21 (2019). https://doi.org/10.1186/s13244-019-0704-z
Symptoms of Glymphatic Obstruction and Paravascular space abnormalities.
Paravascular spaces, also known as perivascular spaces or Virchow-Robin spaces, are fluid-filled spaces that surround blood vessels in the brain. They act as channels for the circulation of cerebrospinal fluid (CSF) and play a role in waste clearance through the glymphatic system. When these spaces become enlarged or disrupted, they can cause symptoms.
Common symptoms associated with paravascular space abnormalities (12)
Cognitive impairment: Enlarged or abnormal paravascular spaces can be associated with cognitive decline, including problems with memory, attention, and executive function.
Headaches: Some individuals with paravascular space abnormalities may experience recurrent or persistent headaches.
Motor and sensory disturbances: In some cases, paravascular space abnormalities can lead to motor and sensory symptoms, such as weakness, numbness, tingling, or difficulty with coordination.
Mood and behavioral changes: This may contribute to mood changes, including depression, anxiety, irritability, or changes in behavior.
Sleep disturbances: Disruption of the glymphatic system due to paravascular space abnormalities can affect sleep patterns, leading to difficulties falling asleep or staying asleep, and poor sleep quality.
Visual disturbances: In rare cases, enlarged or abnormal paravascular spaces can compress adjacent structures and cause visual disturbances, such as blurred vision or visual field deficits.
Enlarged perivascular spaces have also been reported with greater frequency in a variety of settings, albeit generally in smaller size cohorts in autism spectrum disease, cerebral amyloid angiopathy, hypertensive encephalopathy, Parkinsons Disease, SLE and traumatic brain injury.(12)
Symptoms of Glymphatic obstruction are remarkably similar as one might imagine.
Cognitive decline: Obstruction of the glymphatic system can lead to impaired cognitive function, including problems with memory, concentration, and thinking abilities.
Headaches: Persistent or recurrent headaches
Sleep disturbances: Disruption of the glymphatic system can affect sleep patterns, leading to difficulties falling asleep or staying asleep. This can result in poor sleep quality and daytime sleepiness. Zhang et al (10) recent experiments have shown that the glymphatic system is regulated by circadian rhythm rather than the sleep/wake cycle which means that glymphatic system may also be affected by endogenous hormones, not just by sleep/awakening state. However, as glymphatic obstruction may affect the HPA axis, this may be in fact, the reverse.
Mood changes: Glymphatic obstruction can contribute to mood changes, including irritability, depression, anxiety, or changes in emotional stability.
Fatigue: Buildup of waste products in the brain can lead to chronic fatigue, even after adequate rest or sleep.
Balance and coordination problems: Obstruction of the glymphatic system can affect the normal functioning of brain cells, leading to problems with balance, coordination, and motor skills.
Sensory disturbances: Some individuals may experience sensory symptoms such as tingling or numbness in the limbs, altered sensation, or increased sensitivity to light, sound, or touch.
Increased susceptibility to infections: The glymphatic system plays a role in immune function, and its disruption can result in a weakened immune response, making individuals more prone to infections.
Figure 9. Large Numerous Enlarged Perivascular Spaces

Case courtesy of Frank Gaillard, <a href="https://radiopaedia.org/?lang=gb">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/2642?lang=gb">rID: 2642</a>
Glymphatics and Gut-Brain Axis.
Figure 10. The Glymphatic System, neurovascular unit (NVU) and blood-brain barrier

Source: Natale,G et al. Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS. Front. Neurosci. 2021 15:639140. doi: 10.3389/fnins.2021.639140 (4)
Natale et al (4) further describe that a “large body of evidence shows how gastrointestinal pathologies can affect the CNS bypassing or altering blood-brain barrier (BBB) and related pathways, including the glymphatic system. In a novel experimental study a- synuclein fibrils injected into the duodenal and pyloric muscularis layer can spread in the brain, first in the dorsal motor nucleus, and then in the locus coeruleus” and then further.
Glymphatics and Gut-Brain Axis.
Natale et al (4) describe that a “large body of evidence shows how gastrointestinal pathologies can affect the CNS bypassing or altering blood-brain barrier (BBB) and related pathways, including the glymphatic system. In a novel experimental study a- synuclein fibrils injected into the duodenal and pyloric muscularis layer can spread in the brain, first in the dorsal motor nucleus, and then in the locus coeruleus” and then further. Furthermore, “via the microbiota-gut-brain axis, triggering Receptors Expressed on Myeloid cells (TREM)-positive activated macrophages along with inflammatory mediators may reach the brain through blood, glymphatic system, circumventricular organs, or the vagus nerve. This may foster pro-inflammatory reactions in the brain, bridging inflammatory bowel disease and neurological disorders.” This is thought to occur from the SARS-CoV-2 viral infection.
Furthermore, “via the microbiota-gut-brain axis, triggering Receptors Expressed on Myeloid cells (TREM)-positive activated macrophages along with inflammatory mediators may reach the brain through blood, glymphatic system, circumventricular organs, or the vagus nerve. This may foster pro-inflammatory reactions in the brain, bridging inflammatory bowel disease and neurological disorders.” This is thought to occur from the SARS-CoV-2 viral infection. This topic is extensively covered by Natale et al.(4)
Figure 11. Glymphatic Pathway in Pathological conditions.

Source: Natale,G et al. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. 2021. Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS. Front. Neurosci. 15:639140. doi: 10.3389/fnins.2021.639140
Summary
I believe the findings of Boisserand et al (8) and Albayram et al (9) establishes a direct relationship between dysfunctional venous sinus flow and the glymphatic system dysfunction, as seen in increased para-vascular spaces. The potential for autonomic symptoms caused by the sympathetic ganglia that surround the lymph channels is present, but at present unable to be quantified.
Furthermore symptoms of sub-clinical intracranial hypertension (and hypotension) can be caused by the vascular and lymphatic flow abnormalities in the head and neck.
The Glymphatic system’s waste clearance function is affected adversely by Covid and this may potentiate dysfunction when mechanical obstruction is already present. Addressing these mechanical causes can be a major factor in recovering the Long Covid patients who have symptoms reflecting intracranial hypertension and Covid-induced dysautonomia.
Managing POTS and similar conditions has a set of accepted medications that address the symptoms, but I believe it requires attention to the very complex underlying causes to achieve satisfactory control.
References:
1. Wikipedia Contributors. Glymphatic System. 2022. Wikipedia, the Free Encyclopedia. https://en.wikipedia.org/w/index.php?title=Glymphatic_system&oldid=1096466284
2. Xie, L et al. Sleep Drives Metabolite Clearance from the Adult Brain. 2013. Science. https://doi.org/10.1126%2Fscience.1241224
3. Travis J. Miller, Jarom N. Gilstrap, Katsuhide Maeda, Stanley Rockson, Dung H. Nguyen, Correction of complete thoracic duct obstruction with lymphovenous bypass: A case report, Microsurgery, 10.1002/micr.30339, 39, 3, (255-258), (2018).
4. Natale,G et al. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. 2021. Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS. Front. Neurosci. 15:639140. doi: 10.3389/fnins.2021.639140
5. Rasmussen,M., Mestre,H., Nedergaard,M. The glymphatic pathway in neurological disorders. 2018. Lancet Neurology. https://www.thelancet.com/journals/laneur/article/PIIS1474-4422%2818%2930318-1/fulltext
6. Mogensen, F.L.-H.; Delle, C.; Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 2021, 22, 7491. https://doi.org/10.3390/ijms22147491
7. Travis J. Miller, Jarom N. Gilstrap, Katsuhide Maeda, Stanley Rockson, Dung H. Nguyen, Correction of complete thoracic duct obstruction with lymphovenous bypass: A case report, Microsurgery, 10.1002/micr.30339, 39, 3, (255-258), (2018).
8. Jacob, L., Boisserand, L.S.B., Geraldo, L.H.M. et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun 10, 4594 (2019). https://doi.org/10.1038/s41467-019-12568-w
9. Albayram MS, Smith G, Tufan F, Tuna IS, Bostancıklıoğlu M, Zile M, Albayram O. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. 2022 Jan 11;13(1):203. doi: 10.1038/s41467-021-27887-0. PMID: 35017525; PMCID: PMC8752739.
10. Zhang D, Li X, Li B. Glymphatic System Dysfunction in Central Nervous System Diseases and Mood Disorders. Front Aging Neurosci. 2022 Apr 25;14:873697. doi: 10.3389/fnagi.2022.873697. PMID: 35547631; PMCID: PMC9082304.
11. Glia. Wikipedia. https://en.wikipedia.org/wiki/Glia
12. Bell, D. Perivascular Spaces. Radiopedia. https://radiopaedia.org/articles/perivascular-spaces
13. Shen MD. Cerebrospinal fluid and the early brain development of autism. J Neurodev Disord. 2018;10(1):39. Published 2018 Dec 13. https://dx.doi.org/10.1186%2Fs11689-018-9256-7, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=79736902
14. Yamaguchi Y, Wada M, Kimihira L, Nagasawa H. Cognitive impairment due to widespread enlarged perivascular spaces. Radiol Case Rep. 2021 Jul 15;16(9):2640-2645. doi: 10.1016/j.radcr.2021.06.043. PMID: 34345324; PMCID: PMC8319478.
15. Perivascular Space. Wikipedia. https://en.wikipedia.org/wiki/Perivascular_space#:~:text=Other%20general%20symptoms%20associated%20with,%2C%20limb%20weakness%2C%20and%20ataxia.
16. Lee, H. et al. The effect of body posture on brain glymphatic transport. J. Neurosci. 35, 11034–11044 (2015).
17. Bragée B, Michos A, Drum B, Fahlgren M, Szulkin R, Bertilson BC. Signs of Intracranial Hypertension, Hypermobility, and Craniocervical Obstructions in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front Neurol. 2020 Aug 28;11:828. doi: 10.3389/fneur.2020.00828. PMID: 32982905; PMCID: PMC7485557.
18. Hulens M, Rasschaert R, Vansant G, et al. The link between idiopathic intracranial hypertension, fibromyalgia, and chronic fatigue syndrome: exploration of a shared pathophysiology. Journal of pain and research, 2018:11:p3129-3140
19. Lou, J et al. Neuropathology of COVID-19 (neuro-COVID): clinicopathological update. Free Neuropathol. 2021 January 18; 2: . doi:10.17879/freeneuropathology-2021-2993.
20. Midena,E et al. Small Fibre Peripheral Alterations Following COVID-19 Detected by Corneal Confocal Microscopy. J Pers Med. 2022. doi: 10.3390/jpm12040563
21. Abrams,R et al. Small Fiber Neuropathy associated with SARS-CoV-2 Infection. Muscle Nerve. 2022. doi: 10.1002/mus.27458
22. Fard,M et al: Thrombosis in COVID-19 Infection: Role of platelet activation-mediated immunity. Thrombosis Journal (2021) 19:59 https://doi.org/10.1186/s12959-021-00311-9
23. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022. https://doi.org/10.1038/s41591-022-01689-3
24. Dong H, Zhang X, Wang Y, Zhou X, Qian Y, Zhang S. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol Neurobiol. 2017 Mar;54(2):997-1007. doi: 10.1007/s12035-016-9720-x. Epub 2016 Jan 21. PMID: 26797518
25. Wikipedia. Microglia. https://en.wikipedia.org/wiki/Microglia#
26. ANS Balance Assessment. Bioscan Medeia Inc. https://www.bioscan.com/dtr_ans_overview.htm
27. Afrin, Lawrence; Weinstock, Leonard; Molderings, Gerhard. Covid-19 Hyperinflammation and post-Covid 19 may be rooted in Mast Cell Activation Syndrome. 2020: International Journal of Infectious Diseases 100, 327-332.
28. Weinstock,L., et al, Mast cell activation symptoms are prevalent in Long-COVID, 2021. International Journal of Infectious Diseases 112 (2021) 217-226
29. Bashir ST, Redden CR, Raj K, Arcanjo RB, Stasiak S, Li Q, Steelman AJ, Nowak RA. Endometriosis leads to central nervous system-wide glial activation in a mouse model of endometriosis. J Neuroinflammation. 2023 Mar 6;20(1):59. doi: 10.1186/s12974-023-02713-0. PMID: 36879305; PMCID: PMC9987089.
30. Raj V, Haman KL, Raj SR, Byrne D, Blakely RD, Biaggioni I, Robertson D, Shelton RC. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2009 Mar;80(3):339-44. doi: 10.1136/jnnp.2008.144360. Epub 2008 Oct 31. PMID: 18977825; PMCID: PMC2758320.
31. Goldenberg DL. Could Long COVID Research Lead to Breakthroughs in Fibromyalgia and Chronic Fatigue? Practical Pain Manag. 2022. https://www.practicalpainmanagement.com/issue202204/could-long-covid-research-lead-to-breakthroughs-in-understanding
32. Wei,D, Morrison,E. Histology, Astrocytes. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK545142/#:~:text=Astrocytes%20are%20a%20subtype%20of,barrier%2C%20and%20promoting%20synapse%20formation.
33. Huang,S, Fishell,G. In SARS-CoV-2, astrocytes are in for the long haul. PNAS, 2022. https://www.pnas.org/doi/full/10.1073/pnas.2209130119
34. Verger, A., Kas, A., Dudouet, P. et al. Visual interpretation of brain hypometabolism related to neurological long COVID: a French multicentric experience. Eur J Nucl Med Mol Imaging 49, 3197–3202 (2022). https://doi.org/10.1007/s00259-022-05753-5
35. Hotowitz,T, Pellurin,L, Zimmer, E, Guedj,E. Brain fog in long COVID: A glutamatergic hypothesis with astrocyte dysfunction accounting for brain PET glucose hypometabolism. Elsevier, Medical Hypotheses. https://doi.org/10.1016/j.mehy.2023.111186
36. Garland EF, Hartnell IJ, Boche D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front Neurosci. 2022 Feb 16;16:824888. doi: 10.3389/fnins.2022.824888. PMID: 35250459; PMCID: PMC8888691.
37. Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010 Nov 11;468(7321):232-43. doi: 10.1038/nature09613. PMID: 21068832; PMCID: PMC3206737.
38. Song J, da Costa KA, Fischer LM, Kohlmeier M, Kwock L, Wang S, Zeisel SH. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005 Aug;19(10):1266-71. doi: 10.1096/fj.04-3580com. PMID: 16051693; PMCID: PMC1256033.
39. Li,A, Yang,K, Lin,W. Glutamatergic Dysfunction and Glutamatergic Compounds for Major Psychiatric Disorders: Evidence from Clinical Neuroimaging Studies. Front.Psychiatry. 2019. https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00767/full
40. Harris RE. Elevated excitatory neurotransmitter levels in the fibromyalgia brain. Arthritis Res Ther. 2010;12(5):141. doi: 10.1186/ar3136. Epub 2010 Oct 1. PMID: 20959024; PMCID: PMC2991003.
41. Puledda, F et al. Abnormal Glutamatergic and Serotonergic Connectivity in Visual Snow Syndrome and Migraine with Aura. Annals of Neurology. 2023. https://doi.org/10.1002/ana.26745
42. Oya M, Matsuoka K, Kubota M, Fujino J, Tei S, Takahata K, Tagai K, Yamamoto Y, Shimada H, Seki C, Itahashi T, Aoki YY, Ohta H, Hashimoto RI, Sugihara G, Obata T, Zhang MR, Suhara T, Nakamura M, Kato N, Takado Y, Takahashi H, Higuchi M. Increased glutamate and glutamine levels and their relationship to astrocytes and dopaminergic transmissions in the brains of adults with autism. Sci Rep. 2023 Jul 19;13(1):11655. doi: 10.1038/s41598-023-38306-3. PMID: 37468523; PMCID: PMC10356952.
43. Gzielo,K, Nikiforuk,A. Astroglia in Autism Spectrum Disorder. International Journal of Molecular Sciences. 2021. https://doi.org/10.3390/ijms222111544
44. Allen, M., Huang, B.S., Notaras, M.J. et al. Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Mol Psychiatry 27, 2470–2484 (2022). https://doi.org/10.1038/s41380-022-01486-x
45. Nicolaides AN, Morovic S, Menegatti E, Viselner G, Zamboni P. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound: recommendations for a protocol. Funct Neurol. 2011 Oct-Dec;26(4):229-48. PMID: 22364944; PMCID: PMC3814564.
46. Ding J, Guan J, Rajah G, Dornbos D III, Li W, Wang Z, Ding Y, Ji X, Meng R. Clinical and neuroimaging correlates among cohorts of cerebral arteriostenosis, venostenosis and arterio-venous stenosis. Aging (Albany NY). 2019 Dec 2;11(23):11073-11083. doi: 10.18632/aging.102511. Epub 2019 Dec 2. PMID: 31790365; PMCID: PMC6932895.
47. Zhou D, Ding J, Asmaro K, Pan L, Ya J, Yang Q, Fan C, Ding Y, Ji X, Meng R. Clinical Characteristics and Neuroimaging Findings in Internal Jugular Venous Outflow Disturbance. Thromb Haemost. 2019 Feb;119(2):308-318. doi: 10.1055/s-0038-1676815. Epub 2019 Jan 3. PMID: 30605919.
48. Frydrychowski AF, Winklewski PJ, Guminski W (2012) Influence of Acute Jugular Vein Compression on the Cerebral Blood Flow Velocity, Pial Artery Pulsation and Width of Subarachnoid Space in Humans. PLoS ONE 7(10): e48245.
49. Waxenbaum,J, Reddy,V, Varacallo, M. Anatomy, Autonomic Nervous System. StatPearls 2023. https://www.ncbi.nlm.nih.gov/books/NBK539845/#:~:text=The%20autonomic%20nervous%20system%20is,sympathetic%2C%20parasympathetic%2C%20and%20enteric.
50. Bulut,M et al. Decreased Vertebral Artery Hemodynamics in Patients with Loss of Cervical Lordosis. 2016. Med Sci Monit; 22:495-500 https://medscimonit.com/abstract/index/idArt/897500
51. Cole S. Bowdino; Justin Owens; Palma M. Shaw. Anatomy, Abdomen and Pelvis, Renal Veins, 2023. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK538298/
52. Jenssen,N et al. The Glymphatic System- A Beginners’s Guide. Neurochem Res. 2015 December ; 40(12): 2583–2599. doi:10.1007/s11064-015-1581-6.
53. Xie, L et al. Sleep Drives Metabolite Clearance from the Adult Brain. 2013. Science. https://doi.org/10.1126%2Fscience.1241224
54. Mogensen, F.L.-H.; Delle, C.; Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 2021, 22, 7491. https://doi.org/10.3390/ijms22147491
55. Zhang D, Li X, Li B. Glymphatic System Dysfunction in Central Nervous System Diseases and Mood Disorders. Front Aging Neurosci. 2022 Apr 25;14:873697. doi: 10.3389/fnagi.2022.873697. PMID: 35547631; PMCID: PMC9082304.
56. Natale,G et al. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. 2021. Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS. Front. Neurosci. 15:639140. doi: 10.3389/fnins.2021.639140
57. Sullan MJ, Asken BM, Jaffee MS, DeKosky ST, Bauer RM. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci Biobehav Rev. 2018 Jan;84:316-324. doi: 10.1016/j.neubiorev.2017.08.016. Epub 2017 Aug 30. PMID: 28859995.
58. Aaron J. Schain,Agustin Melo-Carrillo, Andrew M. Strassman,Rami Burstein Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci. 2017 Mar 15; 37(11): 2904–2915. doi: 10.1523/JNEUROSCI.3390-16.2017
59. Pu T, Zou W, Feng W, Zhang Y, Wang L, Wang H, Xiao M. Persistent Malfunction of Glymphatic and Meningeal Lymphatic Drainage in a Mouse Model of Subarachnoid Hemorrhage. Exp Neurobiol. 2019 Feb;28(1):104-118. doi: 10.5607/en.2019.28.1.104. Epub 2019 Feb 28. PMID: 30853828; PMCID: PMC6401547.
60. Blitshteyn,S. Is postural orthostatic tachycardia syndrome (POTS) a central nervous system disorder? 2021. Journal of Neurology. https://doi.org/10.1007/s00415-021-10502-z
61. Geddes JR, Ottesen JT, Mehlsen J, Olufsen MS. Postural orthostatic tachycardia syndrome explained using a baroreflex response model. J R Soc Interface. 2022 Aug;19(193):20220220. doi: 10.1098/rsif.2022.0220. Epub 2022 Aug 24. PMID: 36000360; PMCID: PMC9399868.
62. Scholbach, T.: Diagnosis and treatment of vascular compression syndromes of the abdomen based on the anatomical features of man and gender-specific characteristics after puberty. https://scholbach.de/wp-content/uploads/2017/09/20170917-vascular-compression-syndromes-website.pdf
63. Van Campen, C.; Rowe, P.C.; Visser, F.. C Cerebral blood flow remains reduced after tilt testing in myalgic encephalomyelitis/chronic fatigue syndrome patients. Clinical Neurophysiology Practice. 2021. https://doi.org/10.1016/j.cnp.2021.09.001
64. Palpagama TH, Waldvogel HJ, Faull RLM, Kwakowsky A. The Role of Microglia and Astrocytes in Huntington's Disease. Front Mol Neurosci. 2019 Oct 25;12:258. doi: 10.3389/fnmol.2019.00258. PMID: 31708741; PMCID: PMC6824292.
65. Lee KM, Chiu KB, Didier PJ, Baker KC, MacLean AG. Naltrexone treatment reverses astrocyte atrophy and immune dysfunction in self-harming macaques. Brain Behav Immun. 2015 Nov;50:288-297. doi: 10.1016/j.bbi.2015.07.017. Epub 2015 Jul 17. PMID: 26191654; PMCID: PMC4631668.
66. Cabanas,H, Muraki, K,Eaton-Fitch,N, Staines,D, Marshall-Gradisnik,S. Potential Therapeutic Benefit of Low Dose Naltrexone in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Role of Transient Receptor Potential Melastatin 3 Ion Channels in Pathophysiology and Treatment. Frontiers in Immunology. 2021. https://www.frontiersin.org/articles/10.3389/fimmu.2021.687806/full
67. Ioachim, G et al. Altered Pain in the Brainstem and Spinal Cord of Fibromyalgia Patients During the Anticipation and Experience of Experimental Pain. Front. Neurol., 06 May 2022. https://doi.org/10.3389/fneur.2022.862976
68. Benarroch, E.: The locus ceruleus norepinephrine system Functional organization and potential clinical significance. Neurology Nov 2009, 73 (20) 1699-1704; DOI: 10.1212/WNL.0b013e3181c2937c
69. Sullan MJ, Asken BM, Jaffee MS, DeKosky ST, Bauer RM. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci Biobehav Rev. 2018 Jan;84:316-324. doi: 10.1016/j.neubiorev.2017.08.016. Epub 2017 Aug 30. PMID: 28859995.
Comments